Molecular and cellular key players in human islet transplantation
- PMID: 17635636
- PMCID: PMC3922349
- DOI: 10.1111/j.1582-4934.2007.00055.x
Molecular and cellular key players in human islet transplantation
Abstract
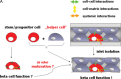
Human islet transplantation could represent an attractive alternative to insulin injections for the treatment of diabetes type 1. However, such an approach requires a better understanding of the molecular and cellular switches controlling ?-cell function in general as well as after transplantation into the liver. Although much research has been done into the suitability of stem or progenitor cells to generate a limitless supply of human ?-cells, a reproducible and efficient protocol for the differentiation of such cells into stably insulin-secreting ?-cells suitable for transplantation has yet to be reported. Fueled by recent findings showing that mature ?-cells are able to regenerate, many efforts have been undertaken to expand this cell pool. Unfortunately, also these approaches had problems to yield sufficiently differentiated human islet cells. The aim of this review is to summarize recent findings describing some of the molecular and cellular key players of islet biology. A more complete understanding of their orchestration and the use of new methods such as real time confocal imaging for the assessment of islet quality may yield the necessary advancements for more successful human islet transplantation.
Figures

References
-
- Humes HD. Cell therapy: leveraging nature's therapeutic potential. J Am Soc Nephrol. 2003;14:2211–3. - PubMed
-
- Dove A. Cell-based therapies go live. Nat Biotechnol. 2002;20:339–43. - PubMed
-
- Brower V. Human ES cells: Can you build a business around them? Nat Biotechnol. 1999;17:139–42. - PubMed
-
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–5. - PubMed
-
- Vassilopoulos G, Russell DW. Cell fusion: An alternative to stem cell plasticity and its therapeutic implications. Curr Opin Genet Dev. 2003;13:480–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

