Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1
- PMID: 17635869
- PMCID: PMC2044541
- DOI: 10.1128/IAI.00532-07
Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1
Abstract
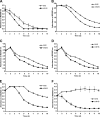
Borrelia spielmanii sp. nov. has recently been shown to be a novel human pathogenic genospecies that causes Lyme disease in Europe. In order to elucidate the immune evasion mechanisms of B. spielmanii, we compared the abilities of isolates obtained from Lyme disease patients and tick isolate PC-Eq17 to escape from complement-mediated bacteriolysis. Using a growth inhibition assay, we show that four B. spielmanii isolates, including PC-Eq17, are serum resistant, whereas a single isolate, PMew, was more sensitive to complement-mediated lysis. All isolates activated complement in vitro, as demonstrated by covalent attachment of C3 fragments; however, deposition of the later activation products C6 and C5b-9 was restricted to the moderately serum-resistant isolate PMew and the serum-sensitive B. garinii isolate G1. Furthermore, serum adsorption experiments revealed that all B. spielmanii isolates acquired the host alternative pathway regulators factor H and factor H-like protein (FHL-1) from human serum. Both complement regulators retained their factor I-mediated C3b inactivation activities when bound to spirochetes. In addition, two distinct factor H and FHL-1 binding proteins, BsCRASP-1 and BsCRASP-2, were identified, which we estimated to be approximately 23 to 25 kDa in mass. A further factor H binding protein, BsCRASP-3, was found exclusively in the tick isolate, PC-Eq17. This is the first report describing an immune evasion mechanism utilized by B. spielmanii sp. nov., and it demonstrates the capture of human immune regulators to resist complement-mediated killing.
Figures






References
-
- Alitalo, A., T. Meri, P. Comstedt, L. Jeffery, J. Tornberg, T. Strandin, H. Lankinen, S. Bergström, M. Cinco, S. R. Vuppala, D. R. Akins, and S. Meri. 2005. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 35:3043-3053. - PubMed
-
- Brade, V., I. Kleber, and G. Acker. 1992. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology 185:453-465. - PubMed
-
- Breitner-Ruddock, S., R. Würzner, J. Schulze, and V. Brade. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. 185:253-260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

