Differential CD28 and inducible costimulatory molecule signaling requirements for protective CD4+ T-cell-mediated immunity against genital tract Chlamydia trachomatis infection
- PMID: 17635872
- PMCID: PMC1951167
- DOI: 10.1128/IAI.00465-07
Differential CD28 and inducible costimulatory molecule signaling requirements for protective CD4+ T-cell-mediated immunity against genital tract Chlamydia trachomatis infection
Abstract
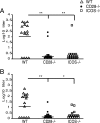
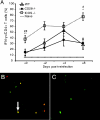
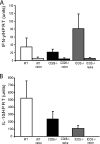
Th1 cells and gamma interferon (IFN-gamma) production play critical roles in protective immunity against genital tract infections by Chlamydia trachomatis. Here we show that inducible costimulatory molecule (ICOS)(-/-) mice develop greatly augmented host resistance against chlamydial infection. Protection following a primary infection was characterized by strong Th1 immunity with enhanced CD4(+) T-cell-mediated IFN-gamma production in the genital tract and high expression of T-bet in the draining para-aortic lymph node. This Th1 dominance was associated with low expression of interleukin 10 (IL-10) mRNA in the uteruses of protected ICOS(-/-) mice. By contrast, CD28(-/-) mice were severely impaired in their adaptive immune response, demonstrating a lack of CD4(+) T cells and IFN-gamma in the genital tract, with a substantial delay in bacterial elimination compared to that seen in wild-type (WT) mice. Upon reinfection, WT mice exhibited a transient local infection with evidence of regulatory T-cell (Treg)/Foxp3 mRNA and a more balanced Th1 and Th2 response in the genital tract than ICOS(-/-) mice, whereas 90% of the latter mice developed sterile immunity, poor expression of local Treg/Foxp3 mRNA, and macroscopic signs of enhanced local immunopathology. Therefore, different requirements for CD28 signaling and ICOS signaling clearly apply to host protection against a genital tract infection by C. trachomatis. Whereas, CD28 signaling is critical, ICOS appears to be dispensable and can have a dampening effect on Th1 development by driving Th2 immunity and anti-inflammation through IL-10 production and promotion of the Foxp3(+) Treg populations in the genital tract. Both the CD28-deficient and the ICOS-deficient mice demonstrated poor specific antibody production, supporting the fact that antibodies are not needed for protection against genital tract chlamydial infections.
Figures
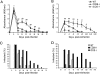


Similar articles
-
Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways.J Immunol. 1997 Apr 1;158(7):3344-52. J Immunol. 1997. PMID: 9120292
-
Th1 cytokine responses fail to effectively control Chlamydia lung infection in ICOS ligand knockout mice.J Immunol. 2010 Apr 1;184(7):3780-8. doi: 10.4049/jimmunol.0901384. Epub 2010 Feb 26. J Immunol. 2010. PMID: 20190137
-
IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection.J Immunol. 1999 Jan 15;162(2):1010-7. J Immunol. 1999. PMID: 9916727
-
Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS).Curr Opin Immunol. 2010 Jun;22(3):326-32. doi: 10.1016/j.coi.2010.01.001. Epub 2010 Jan 29. Curr Opin Immunol. 2010. PMID: 20116985 Review.
-
Regulation of T:B cell interactions by the inducible costimulator molecule: does ICOS "induce" disease?Clin Immunol. 2006 Oct;121(1):13-8. doi: 10.1016/j.clim.2006.04.574. Epub 2006 Jun 21. Clin Immunol. 2006. PMID: 16790364 Review.
Cited by
-
CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract.J Immunol. 2012 Sep 1;189(5):2441-9. doi: 10.4049/jimmunol.1103032. Epub 2012 Aug 1. J Immunol. 2012. PMID: 22855710 Free PMC article.
-
The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection.PLoS Pathog. 2010 Nov 4;6(11):e1001179. doi: 10.1371/journal.ppat.1001179. PLoS Pathog. 2010. PMID: 21079691 Free PMC article.
-
Role of secreted conjunctival mucosal cytokine and chemokine proteins in different stages of trachomatous disease.PLoS Negl Trop Dis. 2008 Jul 16;2(7):e264. doi: 10.1371/journal.pntd.0000264. PLoS Negl Trop Dis. 2008. PMID: 18628987 Free PMC article.
-
ICOS Co-Stimulation: Friend or Foe?Front Immunol. 2016 Aug 10;7:304. doi: 10.3389/fimmu.2016.00304. eCollection 2016. Front Immunol. 2016. PMID: 27559335 Free PMC article. Review.
-
Distinct roles of CD28- and CD40 ligand-mediated costimulation in the development of protective immunity and pathology during Chlamydia muridarum urogenital infection in mice.Infect Immun. 2009 Jul;77(7):3080-9. doi: 10.1128/IAI.00611-08. Epub 2009 Apr 27. Infect Immun. 2009. PMID: 19398542 Free PMC article.
References
-
- Akbari, O., R. H. DeKruyff, and D. T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725-731. - PubMed
-
- Anttila, T., P. Saikku, P. Koskela, A. Bloigu, J. Dillner, I. Ikaheimo, E. Jellum, M. Lehtinen, P. Lenner, T. Hakulinen, A. Narvanen, E. Pukkala, S. Thoresen, L. Youngman, and J. Paavonen. 2001. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 285:47-51. - PubMed
-
- Belkaid, Y., R. B. Blank, and I. Suffia. 2006. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol. Rev. 212:287-300. - PubMed
-
- Bonhagen, K., O. Liesenfeld, M. J. Stadecker, A. Hutloff, K. Erb, A. J. Coyle, M. Lipp, R. A. Kroczek, and T. Kamradt. 2003. ICOS+ Th cells produce distinct cytokines in different mucosal immune responses. Eur. J. Immunol. 33:392-401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

