A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity
- PMID: 17635938
- PMCID: PMC2064450
- DOI: 10.1083/jcb.200705094
A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity
Abstract
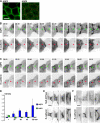
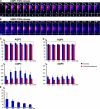
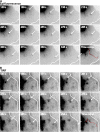
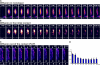
Mechanisms involved in maintaining plasma membrane domains in fully polarized epithelial cells are known, but when and how directed protein sorting and trafficking occur to initiate cell surface polarity are not. We tested whether establishment of the basolateral membrane domain and E-cadherin-mediated epithelial cell-cell adhesion are mechanistically linked. We show that the basolateral membrane aquaporin (AQP)-3, but not the equivalent apical membrane AQP5, is delivered in post-Golgi structures directly to forming cell-cell contacts where it co-accumulates precisely with E-cadherin. Functional disruption of individual components of a putative lateral targeting patch (e.g., microtubules, the exocyst, and soluble N-ethylmaleimide-sensitive factor attachment protein receptors) did not inhibit cell-cell adhesion or colocalization of the other components with E-cadherin, but each blocked AQP3 delivery to forming cell-cell contacts. Thus, components of the lateral targeting patch localize independently of each other to cell-cell contacts but collectively function as a holocomplex to specify basolateral vesicle delivery to nascent cell-cell contacts and immediately initiate cell surface polarity.
Figures
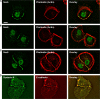
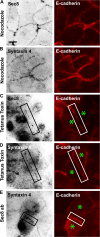
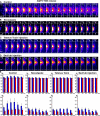
Similar articles
-
Apiconuclear organization of microtubules does not specify protein delivery from the trans-Golgi network to different membrane domains in polarized epithelial cells.Mol Biol Cell. 1998 Mar;9(3):685-99. doi: 10.1091/mbc.9.3.685. Mol Biol Cell. 1998. PMID: 9487135 Free PMC article.
-
Remodeling the cell surface distribution of membrane proteins during the development of epithelial cell polarity.J Cell Biol. 1992 Feb;116(4):889-99. doi: 10.1083/jcb.116.4.889. J Cell Biol. 1992. PMID: 1734022 Free PMC article.
-
Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin.Mol Biol Cell. 2005 Apr;16(4):1744-55. doi: 10.1091/mbc.e04-10-0867. Epub 2005 Feb 2. Mol Biol Cell. 2005. PMID: 15689490 Free PMC article.
-
Epithelial cell surface polarity: the early steps.Front Biosci (Landmark Ed). 2009 Jan 1;14(3):1088-98. doi: 10.2741/3295. Front Biosci (Landmark Ed). 2009. PMID: 19273117 Free PMC article. Review.
-
Generation of epithelial cell polarity: roles for protein trafficking, membrane-cytoskeleton, and E-cadherin-mediated cell adhesion.Cold Spring Harb Symp Quant Biol. 1995;60:763-73. doi: 10.1101/sqb.1995.060.01.082. Cold Spring Harb Symp Quant Biol. 1995. PMID: 8824451 Review. No abstract available.
Cited by
-
A membrane fusion protein αSNAP is a novel regulator of epithelial apical junctions.PLoS One. 2012;7(4):e34320. doi: 10.1371/journal.pone.0034320. Epub 2012 Apr 2. PLoS One. 2012. PMID: 22485163 Free PMC article.
-
Polarized transport of membrane and secreted proteins during lumen morphogenesis.Semin Cell Dev Biol. 2023 Jan 15;133:65-73. doi: 10.1016/j.semcdb.2022.03.016. Epub 2022 Mar 17. Semin Cell Dev Biol. 2023. PMID: 35307284 Free PMC article. Review.
-
Mechanisms of cell polarity and aquaporin sorting in the nephron.Pflugers Arch. 2011 Jun;461(6):607-21. doi: 10.1007/s00424-011-0928-3. Epub 2011 Feb 16. Pflugers Arch. 2011. PMID: 21327781 Review.
-
Molecular components of the adherens junction.Biochim Biophys Acta. 2008 Mar;1778(3):562-71. doi: 10.1016/j.bbamem.2007.12.015. Epub 2008 Jan 14. Biochim Biophys Acta. 2008. PMID: 18206110 Free PMC article. Review.
-
Cadherins and cancer: how does cadherin dysfunction promote tumor progression?Oncogene. 2008 Nov 24;27(55):6920-9. doi: 10.1038/onc.2008.343. Oncogene. 2008. PMID: 19029934 Free PMC article. Review.
References
-
- Brunger, A.T. 2005. Structure and function of SNARE and SNARE-interacting proteins. Q. Rev. Biophys. 38:1–47. - PubMed
-
- Chang, J.T., V.R. Palanivel, I. Kinjyo, F. Schambach, A.M. Intlekofer, A. Banerjee, S.A. Longworth, K.E. Vinup, P. Mrass, J. Oliaro, et al. 2007. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 315:1687–1691. - PubMed

