Structural basis of GLUT1 inhibition by cytoplasmic ATP
- PMID: 17635959
- PMCID: PMC2031153
- DOI: 10.1085/jgp.200709818
Structural basis of GLUT1 inhibition by cytoplasmic ATP
Abstract
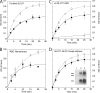
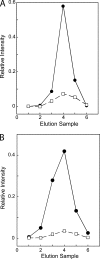
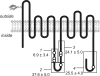
Cytoplasmic ATP inhibits human erythrocyte glucose transport protein (GLUT1)-mediated glucose transport in human red blood cells by reducing net glucose transport but not exchange glucose transport (Cloherty, E.K., D.L. Diamond, K.S. Heard, and A. Carruthers. 1996. Biochemistry. 35:13231-13239). We investigated the mechanism of ATP regulation of GLUT1 by identifying GLUT1 domains that undergo significant conformational change upon GLUT1-ATP interaction. ATP (but not GTP) protects GLUT1 against tryptic digestion. Immunoblot analysis indicates that ATP protection extends across multiple GLUT1 domains. Peptide-directed antibody binding to full-length GLUT1 is reduced by ATP at two specific locations: exofacial loop 7-8 and the cytoplasmic C terminus. C-terminal antibody binding to wild-type GLUT1 expressed in HEK cells is inhibited by ATP but binding of the same antibody to a GLUT1-GLUT4 chimera in which loop 6-7 of GLUT1 is substituted with loop 6-7 of GLUT4 is unaffected. ATP reduces GLUT1 lysine covalent modification by sulfo-NHS-LC-biotin by 40%. AMP is without effect on lysine accessibility but antagonizes ATP inhibition of lysine modification. Tandem electrospray ionization mass spectrometry analysis indicates that ATP reduces covalent modification of lysine residues 245, 255, 256, and 477, whereas labeling at lysine residues 225, 229, and 230 is unchanged. Exogenous, intracellular GLUT1 C-terminal peptide mimics ATP modulation of transport whereas C-terminal peptide-directed IgGs inhibit ATP modulation of glucose transport. These findings suggest that transport regulation involves ATP-dependent conformational changes in (or interactions between) the GLUT1 C terminus and the C-terminal half of GLUT1 cytoplasmic loop 6-7.
Figures




References
-
- Abramson, J., I. Smirnova, V. Kasho, G. Verner, H.R. Kaback, and S. Iwata. 2003. Structure and mechanism of the lactose permease of Escherichia coli. Science. 301:610–615. - PubMed
-
- Appleman, J.R., and G.E. Lienhard. 1985. Rapid kinetics of the glucose transporter from human erythrocytes. Detection and measurement of a half-turnover of the purified transporter. J. Biol. Chem. 260:4575–4578. - PubMed
-
- Baldwin, S.A., J.M. Baldwin, F.R. Gorga, and G.E. Lienhard. 1979. Purification of the cytochalasin B binding component of the human erythrocyte monosaccharide transport system. Biochim. Biophys. Acta. 552:183–188. - PubMed
-
- Baldwin, S.A., J.M. Baldwin, and G.E. Lienhard. 1982. The monosaccharide transporter of the human erythrocyte. Characterization of an improved preparation. Biochemistry. 21:3836–3842. - PubMed
-
- Blodgett, D.M., and A. Carruthers. 2005. Quench-flow analysis reveals multiple phases of GluT1-mediated sugar transport. Biochemistry. 44:2650–2660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

