An in vivo model of apoptosis: linking cell behaviours and caspase substrates in embryos lacking DIAP1
- PMID: 17636001
- PMCID: PMC7617992
- DOI: 10.1242/jcs.03472
An in vivo model of apoptosis: linking cell behaviours and caspase substrates in embryos lacking DIAP1
Abstract
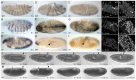
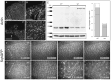
The apoptotic phenotype is characterised by dynamic changes in cell behaviours such as cell rounding and blebbing, followed by chromatin condensation and cell fragmentation. Whereas the biochemical pathways leading to caspase activation have been actively studied, much less is known about how caspase activity changes cell behaviours during apoptosis. Here, we address this question using early Drosophila melanogaster embryos lacking DIAP1. Reflecting its central role in the inhibition of apoptosis, loss of DIAP1 causes massive caspase activation. We generated DIAP1-depleted embryos by either using homozygous null mutants for thread, the gene coding DIAP1, or by ectopically expressing in early embryos the RGH protein Reaper, which inhibits DIAP1. We show that (1) all cells in embryos lacking DIAP1 follow synchronously the stereotypic temporal sequence of behaviours described for apoptotic mammalian cells and (2) these cell behaviours specifically require caspase activity and are not merely a consequence of cellular stress. Next, we analyse the dynamic changes in the localisation of actomyosin, Discs large, Bazooka and DE-cadherin in the course of apoptosis. We show that early changes in Bazooka and Discs large correlate with early processing of these proteins by caspases. DE-cadherin and Myosin light chain do not appear to be cleaved, but their altered localisation can be explained by cleavage of known regulators. This illustrates how embryos lacking DIAP1 can be used to characterise apoptotic changes in the context of an embryo, thus providing an unprecedented in vivo model in which thousands of cells initiate apoptosis simultaneously.
Figures




References
-
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117:29–43. - PubMed
-
- Adrain C, Creagh EM, Cullen SP, Martin SJ. Caspase-dependent inactivation of proteasome function during programmed cell death in Drosophila and man. J Biol Chem. 2004;279:36923–36930. - PubMed
-
- Adrain C, Brumatti G, Martin SJ. Apoptosomes: protease activation platforms to die from. Trends Biochem Sci. 2006;31:243–247. - PubMed
-
- Allen MJ, O’Kane CJ, Moffat KG. Cell ablation using wild-type and cold-sensitive ricin-A chain in Drosophila embryonic mesoderm. Genesis. 2002;34:132–134. - PubMed
-
- Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol. 2001;3:50–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

