The L7Ae RNA binding motif is a multifunctional domain required for the ribosome-dependent Sec incorporation activity of Sec insertion sequence binding protein 2
- PMID: 17636016
- PMCID: PMC2099609
- DOI: 10.1128/MCB.00632-07
The L7Ae RNA binding motif is a multifunctional domain required for the ribosome-dependent Sec incorporation activity of Sec insertion sequence binding protein 2
Abstract
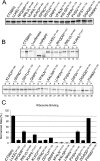
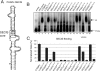
The decoding of specific UGA codons as selenocysteine is specified by the Sec insertion sequence (SECIS) element. Additionally, Sec-tRNA([Ser]Sec) and the dedicated Sec-specific elongation factor eEFSec are required but not sufficient for nonsense suppression. SECIS binding protein 2 (SBP2) is also essential for Sec incorporation, but its precise role is unknown. In addition to binding the SECIS element, SBP2 binds stably and quantitatively to ribosomes. To determine the function of the SBP2-ribosome interaction, conserved amino acids throughout the SBP2 L7Ae RNA binding motif were mutated to alanine in clusters of five. Mutant proteins were analyzed for ribosome binding, SECIS element binding, and Sec incorporation activity, allowing us to identify two distinct but interdependent sites within the L7Ae motif: (i) a core L7Ae motif required for SECIS binding and ribosome binding and (ii) an auxiliary motif involved in physical and functional interactions with the ribosome. Structural modeling of SBP2 based on the 15.5-kDa protein-U4 snRNA complex strongly supports a two-site model for L7Ae domain function within SBP2. These results provide evidence that the SBP2-ribosome interaction is essential for Sec incorporation.
Figures
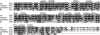




References
-
- Ban, N., P. Nissen, J. Hansen, P. B. Moore, and T. A. Steitz. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289:905-920. - PubMed
-
- Chao, J. A., and J. R. Williamson. 2004. Joint X-ray and NMR refinement of the yeast L30e-mRNA complex. Structure 12:1165-1176. - PubMed
-
- Chavatte, L., B. A. Brown, and D. M. Driscoll. 2005. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat. Struct. Mol. Biol. 12:408-416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
