Phosphorylation of Slx4 by Mec1 and Tel1 regulates the single-strand annealing mode of DNA repair in budding yeast
- PMID: 17636031
- PMCID: PMC2099619
- DOI: 10.1128/MCB.00135-07
Phosphorylation of Slx4 by Mec1 and Tel1 regulates the single-strand annealing mode of DNA repair in budding yeast
Abstract
Budding yeast (Saccharomyces cerevisiae) Slx4 is essential for cell viability in the absence of the Sgs1 helicase and for recovery from DNA damage. Here we report that cells lacking Slx4 have difficulties in completing DNA synthesis during recovery from replisome stalling induced by the DNA alkylating agent methyl methanesulfonate (MMS). Although DNA synthesis restarts during recovery, cells are left with unreplicated gaps in the genome despite an increase in translesion synthesis. In this light, epistasis experiments show that SLX4 interacts with genes involved in error-free bypass of DNA lesions. Slx4 associates physically, in a mutually exclusive manner, with two structure-specific endonucleases, Rad1 and Slx1, but neither of these enzymes is required for Slx4 to promote resistance to MMS. However, Rad1-dependent DNA repair by single-strand annealing (SSA) requires Slx4. Strikingly, phosphorylation of Slx4 by the Mec1 and Tel1 kinases appears to be essential for SSA but not for cell viability in the absence of Sgs1 or for cellular resistance to MMS. These results indicate that Slx4 has multiple functions in responding to DNA damage and that a subset of these are regulated by Mec1/Tel1-dependent phosphorylation.
Figures
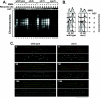


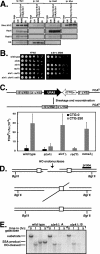
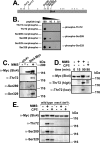
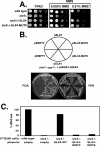

References
-
- Beranek, D. T. 1990. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. 231:11-30. - PubMed
-
- Brusky, J., Y. Zhu, and W. Xiao. 2000. UBC13, a DNA-damage-inducible gene, is a member of the error-free postreplication repair pathway in Saccharomyces cerevisiae. Curr. Genet. 37:168-174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
