Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence
- PMID: 17636116
- PMCID: PMC1941508
- DOI: 10.1073/pnas.0705313104
Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence
Abstract
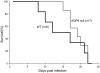
Human and rodent erythrocytes are known to be highly permeable to glycerol. Aquaglyceroporin aquaporin (AQP)3 is the major glycerol channel in human and rat erythrocytes. However, AQP3 expression has not been observed in mouse erythrocytes. Here we report the presence of an aquaglyceroporin, AQP9, in mouse erythrocytes. AQP9 levels rise as reticulocytes mature into erythrocytes and as neonatal pups develop into adult mice. Mice bearing targeted disruption of both alleles encoding AQP9 have erythrocytes that appear morphologically normal. Compared with WT cells, erythrocytes from AQP9-null mice are defective in rapid glycerol transport across the cell membrane when measured by osmotic lysis, [(14)C]glycerol uptake, or stopped-flow light scattering. In contrast, the water and urea permeabilities are intact. Although the physiological role of glycerol in the normal function of erythrocytes is not clear, plasma glycerol is an important substrate for lipid biosynthesis of intraerythrocytic malarial parasites. AQP9-null mice at the age of 4 months infected with Plasmodium berghei survive longer during the initial phase of infection compared with WT mice. We conclude that AQP9 is the major glycerol channel in mouse erythrocytes and suggest that this transport pathway may contribute to the virulence of intraerythrocytic stages of malarial infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Jammed traffic impedes parasite growth.Proc Natl Acad Sci U S A. 2007 Aug 28;104(35):13855-6. doi: 10.1073/pnas.0706632104. Epub 2007 Aug 20. Proc Natl Acad Sci U S A. 2007. PMID: 17709740 Free PMC article. No abstract available.
References
-
- Preston GM, Carroll TP, Guggino WB, Agre P. Science. 1992;256:385–387. - PubMed
-
- Wessels JM, Veerkamp JH. Biochim Biophys Acta. 1973;291:190–196. - PubMed
-
- Roudier N, Verbavatz JM, Maurel C, Ripoche P, Tacnet F. J Biol Chem. 1998;273:8407–8412. - PubMed
-
- Yang B, Ma T, Verkman AS. J Biol Chem. 2001;276:624–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

