The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization
- PMID: 17636121
- PMCID: PMC1941497
- DOI: 10.1073/pnas.0703310104
The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization
Abstract
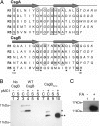
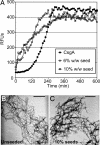
Curli are functional amyloid fibers assembled by enteric bacteria such as Escherichia coli and Salmonella spp. In E. coli, the polymerization of the major curli fiber subunit protein CsgA into an amyloid fiber depends on the minor curli subunit protein, CsgB. The outer membrane-localized CsgB protein shares approximately 30% sequence identity with the amyloid-forming protein CsgA, suggesting that CsgB might also have amyloidogenic properties. Here, we characterized the biochemical properties of CsgB and the molecular basis for CsgB-mediated nucleation of CsgA. Deletion analysis revealed that a CsgB molecule missing 19 amino acids from its C terminus (CsgB(trunc)) was not outer membrane-associated, but secreted away from the cell. CsgB(trunc) was overexpressed and purified from the extracellular milieu of cells as an SDS-soluble, nonaggregated protein. Soluble CsgB(trunc) assembled into fibers that bound to the amyloid-specific dyes Congo red and thioflavin-T. CsgB(trunc) fibers were able to seed soluble CsgA polymerization in vitro. CsgB(trunc) displayed modest nucleator activity in vivo, as demonstrated by its ability to convert extracellular CsgA into an amyloid fiber. Unlike WT CsgB, CsgB(trunc) was only able to act as a nucleator when cells were genetically manipulated to secrete higher concentrations of CsgA. This work represents a unique demonstration of functional amyloid nucleation and it suggests an elegant model for how E. coli guides efficient amyloid fiber formation on the cell surface.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
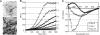
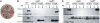
Similar articles
-
The C-terminal repeating units of CsgB direct bacterial functional amyloid nucleation.J Mol Biol. 2012 Sep 21;422(3):376-89. doi: 10.1016/j.jmb.2012.05.043. Epub 2012 Jun 7. J Mol Biol. 2012. PMID: 22684146 Free PMC article.
-
Role of Escherichia coli curli operons in directing amyloid fiber formation.Science. 2002 Feb 1;295(5556):851-5. doi: 10.1126/science.1067484. Science. 2002. PMID: 11823641 Free PMC article.
-
The E. coli CsgB nucleator of curli assembles to β-sheet oligomers that alter the CsgA fibrillization mechanism.Proc Natl Acad Sci U S A. 2012 Apr 24;109(17):6502-7. doi: 10.1073/pnas.1204161109. Epub 2012 Apr 9. Proc Natl Acad Sci U S A. 2012. PMID: 22493266 Free PMC article.
-
Curli provide the template for understanding controlled amyloid propagation.Prion. 2008 Apr-Jun;2(2):57-60. doi: 10.4161/pri.2.2.6746. Epub 2008 Apr 5. Prion. 2008. PMID: 19098444 Free PMC article. Review.
-
Bacterial amyloid formation: structural insights into curli biogensis.Trends Microbiol. 2015 Nov;23(11):693-706. doi: 10.1016/j.tim.2015.07.010. Epub 2015 Oct 1. Trends Microbiol. 2015. PMID: 26439293 Free PMC article. Review.
Cited by
-
Amyloidogenic peptides of yeast cell wall glucantransferase Bgl2p as a model for the investigation of its pH-dependent fibril formation.Prion. 2013 Mar-Apr;7(2):175-84. doi: 10.4161/pri.22992. Epub 2012 Dec 3. Prion. 2013. PMID: 23208381 Free PMC article.
-
Microbial manipulation of the amyloid fold.Res Microbiol. 2012 Nov-Dec;163(9-10):592-606. doi: 10.1016/j.resmic.2012.10.009. Epub 2012 Oct 27. Res Microbiol. 2012. PMID: 23108148 Free PMC article. Review.
-
Rubber elongation factor (REF), a major allergen component in Hevea brasiliensis latex has amyloid properties.PLoS One. 2012;7(10):e48065. doi: 10.1371/journal.pone.0048065. Epub 2012 Oct 25. PLoS One. 2012. PMID: 23133547 Free PMC article.
-
Bacterial amyloids.Methods Mol Biol. 2012;849:303-20. doi: 10.1007/978-1-61779-551-0_21. Methods Mol Biol. 2012. PMID: 22528099 Free PMC article.
-
Reducing the Amyloidogenicity of Functional Amyloid Protein FapC Increases Its Ability To Inhibit α-Synuclein Fibrillation.ACS Omega. 2019 Feb 22;4(2):4029-4039. doi: 10.1021/acsomega.8b03590. eCollection 2019 Feb 28. ACS Omega. 2019. PMID: 31459612 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

