Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells
- PMID: 17636128
- PMCID: PMC1941479
- DOI: 10.1073/pnas.0703787104
Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells
Abstract
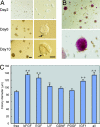
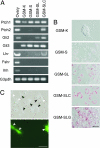
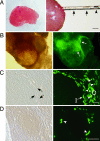
Although ovarian theca cells play an indispensable role in folliculogenesis by providing follicular structural integrity and steroid substrates for estrogen production, little information is available about their recruitment, growth, and differentiation because their immature forms have not been identified. We have isolated putative thecal stem cells with the ability to self-renew and differentiate in vivo and in vitro. They are similar to fibroblasts in morphology and proliferate in vitro as round colonies with a homogenous cell population. They were induced to differentiate into early precursors and steroidogenic cells in a stepwise manner after treatment with serum, luteinizing hormone, and paracrine factors from granulosa cells. At each differentiation step, these cells displayed appropriate gene expression and morphological markers and later secreted androstenedione. The fully mature morphology was achieved by coculture with isolated granulosa cells. When transplanted into the ovaries, the putative thecal stem cells colonized exclusively in the ovarian interstitium and the thecal layer of follicles as differentiated cells. Thus, thecal stem cells appear to be present in neonatal ovaries and can be isolated, purified, and induced to differentiate in vitro. Thecal stem cells could provide an invaluable in vitro experimental system to study interactions among the oocytes, granulosa cells, and theca cells during normal folliculogenesis and to study ovarian pathology caused by theca cell dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Magoffin DA. Int J Biochem Cell Biol. 2005;37:1344–1349. - PubMed
-
- Demeestere I, Centner J, Gervy C, Englert Y, Delbaere A. Reproduction (Bristol, UK) 2005;130:147–156. - PubMed
-
- Hirshfield AN. Biol Reprod. 1991;44:1157–1162. - PubMed
-
- Lo KC, Lei Z, Rao Ch V, Beck J, Lamb DJ. Endocrinology. 2004;145:4011–4015. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

