Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study
- PMID: 17636329
- PMCID: PMC12161746
- DOI: 10.1007/s00432-007-0250-9
Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study
Abstract
Background: Cardiotoxicity associated with 5-Fluorouracil (5FU) administration has been infrequently reported in literature, albeit various series of acute coronary syndromes have recorded a low but definite incidence of the above toxicity. In the present study, patients undergoing 5FU-based and oral capecitabine (Xeloda-based chemotherapy were tested for the potential development of cardiac-related symptoms during their administration.
Patients and methods: Six hundred and forty-four patients entered the study. Those experiencing any cardiac-related symptoms during 5FU infusion or oral capecitabine were subjected to ECG and serum cardiac enzymes determination. If cardiotoxicity was confirmed, 5FU infusion or oral capecitabine were interrupted, sublingual nitrates administered and cardiac monitoring initiated, while patients with >two-fold enzyme elevation were followed in a coronary care unit for at least 72 h. Cases with acute myocardial infarction were excluded from further 5FU or oral capecitabine treatment.
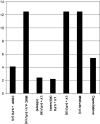
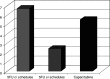
Results: Overall 26 patients (4.03%) developed symptoms and/or ECG abnormalities due to 5FU and capecitabine. Patients with continuous 5FU infusion presented a higher incidence of cardiotoxicity [14/209; 6.7%, 95% confidence interval (CI) = 3.3-10.1%] than the remaining (7/317; 2.3%, 95% CI = 0.8-3.3%) (P < 0.012). Specifically, an increased incidence of cardiac-related events was encountered in patients with continuous 24-h 5FU + LV infusion for 5 days (12.5%, 95% CI = 2.3-22.7%) rather than in patients with the same schedule without LV (5.3%, 95% CI = 1.95-8.67%) (P < 0.027), as well as in patients with short 5FU + LV administration (2.4%, 95% CI = 0.9-3.9%) (P < 0.019). Overall, 3/54 patients (5.5%, 95% CI = -0.6-11.1%) on oral capecitabine developed cardiac-related events. Seven out of the 20 patients suffered an acute myocardial infarction, 6 developed ischemia only, while 4 more patients had ECG consistent with coronary vasospasm and 3 with conduction disturbances, of which one subsequently died. Patients administered oral capecitabine had a similar incidence of cardiac-related events; 1/22 (4.5%) patients with advanced breast cancer and 2/32 (6.2%) with colorectal cancer.
Conclusions: The present study supports the toxic effect of 5-FU on the myocardium, which is largely schedule-dependent, whereas a low but finite risk of such toxicity has been observed with oral capecitabine. A high level of alertness is required when using fluoropyrimidines (i.v. 5FU or oral capecitabine), while their toxic effect on the coronary endothelium and myocardium merits further investigation.
Figures
References
-
- Akhtar SS, Salim KP, Bano ZA (1993) Symptomatic cardiotoxicity with high-dose 5-fluorouracil infusion: a prospective study. Oncology 50:441–444 - PubMed
-
- Blum RH, Carter SK (1974) Adriamycin: a new anticancer drug with significant clinical activicy. Ann Intern Med 80:249–259 - PubMed
-
- Cwikiel M, Zhang B, Eskilsson J, Wieslander JB, Albertsson M (1995) The influence of 5-fluorouracil on the endothelium in small arteries. An electron microscopic study in rabbits. Scanning Microsc 9:561–576 - PubMed
-
- Cwikiel M, Eskilsson J, Albertsson M, Stavenow L (1996a) The influence of 5-fluorouracil and methotrexate on vascular endothelium. An experimental study using endothelial cells in the culture. Ann Oncol 7:731–737 - PubMed
-
- Cwikiel M, Eskilsson J, Wieslander JB, Stjernquist U, Albertsson M (1996b) The appearance of endothelium in small arteries after the treatment with 5-fluorouracil. An electron microscopic study of late effects in rabbits. Scan Micros 10:187–192 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

