Effect of thrombin peptide 508 (TP508) on bone healing during distraction osteogenesis in rabbit tibia
- PMID: 17636332
- PMCID: PMC2039796
- DOI: 10.1007/s00441-007-0448-9
Effect of thrombin peptide 508 (TP508) on bone healing during distraction osteogenesis in rabbit tibia
Abstract
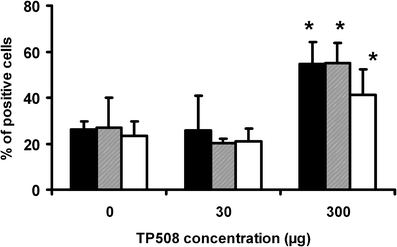
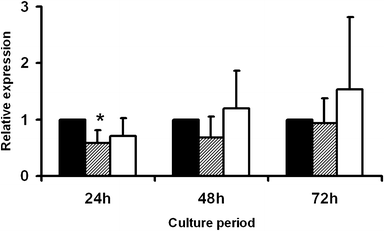
Thrombin-related peptide 508 (TP508) accelerates bone regeneration during distraction osteogenesis (DO). We have examined the effect of TP508 on bone regeneration during DO by immunolocalization of Runx2 protein, a marker of osteoblast differentiation, and of osteopontin (OPN) and bone sialoprotein (BSP), two late markers of the osteoblast lineage. Distraction was performed in tibiae of rabbits over a period of 6 days. TP508 (30 or 300 microg) or vehicle was injected into the distraction gap at the beginning and end of the distraction period. Two weeks after active distraction, tissue samples were harvested and processed for immunohistochemical analysis. We also tested the in vitro effect of TP508 on Runx2 mRNA expression in osteoblast-like (MC3T3-E1) cells by polymerase chain reaction analysis. Runx2 and OPN protein were observed in preosteoblasts, osteoblasts, osteocytes of newly formed bone, blood vessel cells and many fibroblast-like cells of the soft connective tissue. Immunostaining for BSP was more restricted to osteoblasts and osteocytes. Significantly more Runx2- and OPN-expressing cells were seen in the group treated with 300 microg TP508 than in the control group injected with saline or with 30 microg TP508. However, TP508 failed to increase Runx2 mRNA levels significantly in MC3T3-E1 cells after 2-3 days of exposure. Our data suggest that TP508 enhances bone regeneration during DO by increasing the proportion of cells of the osteoblastic lineage. Clinically, TP508 may shorten the healing time during DO; this might be of benefit when bone regeneration is slow.
Figures



Similar articles
-
Bone formation is enhanced by thrombin-related peptide TP508 during distraction osteogenesis.J Orthop Res. 2005 Jan;23(1):196-202. doi: 10.1016/j.orthres.2004.05.006. J Orthop Res. 2005. PMID: 15607893
-
Local injection of thrombin-related peptide (TP508) in PPF/PLGA microparticles-enhanced bone formation during distraction osteogenesis.J Orthop Res. 2008 Apr;26(4):539-46. doi: 10.1002/jor.20495. J Orthop Res. 2008. PMID: 17960653
-
TP508 Promotes Bone Regeneration on Distraction Osteogenesis via the Activation of Wnt/β-catenin Signaling Pathway.Curr Pharm Biotechnol. 2025;26(3):402-410. doi: 10.2174/0113892010289575240306033011. Curr Pharm Biotechnol. 2025. PMID: 38468521
-
Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues.J Bone Joint Surg Am. 2006 Nov;88 Suppl 3:132-9. doi: 10.2106/JBJS.F.00892. J Bone Joint Surg Am. 2006. PMID: 17079379 Review.
-
Thrombin peptide, TP508, stimulates angiogenic responses in animal models of dermal wound healing, in chick chorioallantoic membranes, and in cultured human aortic and microvascular endothelial cells.Gen Pharmacol. 2000 Nov;35(5):249-54. doi: 10.1016/s0306-3623(01)00118-5. Gen Pharmacol. 2000. PMID: 11888680 Review.
Cited by
-
Accumulation of type VI collagen in the primary osteon of the rat femur during postnatal development.J Anat. 2015 May;226(5):478-88. doi: 10.1111/joa.12296. Epub 2015 May 5. J Anat. 2015. PMID: 25943007 Free PMC article.
-
The role of peptides in bone healing and regeneration: a systematic review.BMC Med. 2016 Jul 11;14:103. doi: 10.1186/s12916-016-0646-y. BMC Med. 2016. PMID: 27400961 Free PMC article.
-
Inhibin A enhances bone formation during distraction osteogenesis.J Orthop Res. 2012 Feb;30(2):288-95. doi: 10.1002/jor.21501. Epub 2011 Aug 1. J Orthop Res. 2012. PMID: 21809377 Free PMC article.
-
Bone regeneration during distraction osteogenesis.Odontology. 2009 Jul;97(2):63-75. doi: 10.1007/s10266-009-0101-z. Epub 2009 Jul 29. Odontology. 2009. PMID: 19639448 Review.
-
Medical countermeasures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. report of an NIAID Workshop, March 26-27, 2007.Radiat Res. 2008 Jun;169(6):712-21. doi: 10.1667/RR1295.1. Radiat Res. 2008. PMID: 18494548 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1600-0501.2005.01231.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1600-0501.2005.01231.x'}, {'type': 'PubMed', 'value': '16907773', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16907773/'}]}
- Amir LR, Becking AG, Jovanovic A, Perdijk FBT, Everts V, Bronckers AL (2006) Vertical distraction osteogenesis in the human mandible: a prospective morphometric study. Clin Oral Implants Res 17:417–425 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/0026-2862(92)90004-9', 'is_inner': False, 'url': 'https://doi.org/10.1016/0026-2862(92)90004-9'}, {'type': 'PubMed', 'value': '1608338', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1608338/'}]}
- Belloni PN, Carney DH, Nicolson GL (1992) Organ-derived microvessel endothelial cells exhibit differential responsiveness to thrombin and other growth factors. Microvasc Res 43:20–45 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0925-4773(00)00562-1', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0925-4773(00)00562-1'}, {'type': 'PubMed', 'value': '11231086', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11231086/'}]}
- Bronckers AL, Engelse MA, Cavender A, Gaikwad J, D’Souza RN (2001) Cell-specific patterns of Cbfa1 mRNA and protein expression in postnatal murine dental tissues. Mech Dev 101:255–258 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1359/JBMR.041118', 'is_inner': False, 'url': 'https://doi.org/10.1359/jbmr.041118'}, {'type': 'PubMed', 'value': '15746987', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15746987/'}]}
- Bronckers AL, Sasaguri K, Cavender AC, D’Souza RN, Engelse MA (2005) Expression of Runx2/Cbfa1/Pebp2alphaA during angiogenesis in postnatal rodent and fetal human orofacial tissues. J Bone Miner Res 20:428–437 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1073/pnas.72.1.131', 'is_inner': False, 'url': 'https://doi.org/10.1073/pnas.72.1.131'}, {'type': 'PMC', 'value': 'PMC432255', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC432255/'}, {'type': 'PubMed', 'value': '1054489', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1054489/'}]}
- Chen LB, Buchanan JM (1975) Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci USA 72:131–135 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

