Antipsychotic medication for childhood-onset schizophrenia
- PMID: 17636744
- PMCID: PMC6984668
- DOI: 10.1002/14651858.CD004027.pub2
Antipsychotic medication for childhood-onset schizophrenia
Abstract
Background: Childhood-onset schizophrenia is schizophrenia with onset prior to the age of 13 years. Although it is rare, people who suffer from schizophrenia at an early age appear to have a clinically severe form of the illness with poor long-term prognosis. Antipsychotic medication is one way of managing this rare but serious mental illness.
Objectives: To examine the effects of antipsychotic medication for childhood-onset schizophrenia.
Search strategy: We searched the Cochrane Schizophrenia Group Trials Register (November 2006 and February 2007), inspected references of all identified studies for further trials and contacted relevant pharmaceutical companies and authors of trials for additional information.
Selection criteria: We included all randomised clinical trials involving children and young people with a diagnosis of childhood onset schizophrenia (i.e. with a diagnosis of schizophrenia before the age of 13) comparing any antipsychotic drug with another antipsychotic or placebo.
Data collection and analysis: We reliably selected, quality assessed and extracted data from trials. We excluded data where more than 50% of participants in any group were lost to follow up. For homogenous dichotomous data we calculated random effects, relative risk (RR) and its 95% confidence interval (CI) and, where appropriate, number needed to treat (NNT) on an intention-to-treat basis. For normal continuous data we calculated the weighted mean difference (WMD).
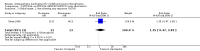
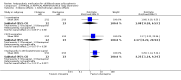
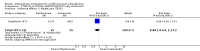
Main results: From a total of 2062 citations, we identified six relevant trials. We categorised trials into three comparisons: atypical versus typical, atypical versus atypical and typical versus typical antipsychotic drugs. The only comparison to find any differences between treatment groups was atypical versus typical antipsychotic drugs. A few results from one study favoured the atypical antipsychotic clozapine over haloperidol in treating treatment resistant childhood-onset schizophrenia (n=21, WMD CGAS 17.00 CI 7.74 to 26.26; n=21, WMD Bunney-Hamburg Psychosis Rating Scale -3.60 CI -6.64 to -0.56). Participants on clozapine, however, were three times more likely to have drowsiness (1 RCT, n=21, RR 3.30 CI 1.23 to 8.85, NNH 2 CI 2 to 17) and half of the children receiving clozapine had neutropenia (1 RCT, n=21, RR 12, CI 0.75 to 192.86).
Authors' conclusions: There are few relevant trials and, presently, there is little conclusive evidence regarding the effects of antipsychotic medication for those with early onset schizophrenia. Some benefits were identified in using the atypical antipsychotic clozapine compared with haloperidol but the benefits were offset by an increased risk of serious adverse effects. Larger, more robust, trials are required.
Conflict of interest statement
Eilis Kennedy ‐ None known Ajit Kumar ‐ None known Soumitra Shankar Datta ‐ None known
Figures



























Update of
References
References to studies included in this review
Engelhardt 1973 {published data only}
-
- Engelhardt DM, Polizos P, Waizer J, Hoffman SP. A double blind comparison of fluphenazine and haloperidol in outpatient schizophrenic children. Journal of Autism and Childhood Schizophrenia 1973;3(2):128‐37. [PsycINFO 52‐03645] - PubMed
Faretra 1970 {published data only}
-
- Faretra G, Dooher L, Dowling J. Comparison of haloperidol and fluphenazine in disturbed children. American Journal of Psychiatry 1970;126:1670‐3. [MEDLINE: ] - PubMed
Kumra 1996 {published data only}
-
- Huffman GB. Efficacy of clozapine for schizophrenia in children. American Family Physician 1997;55(4):1356. [MEDLINE: ]
-
- Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, Hamburger SD, Smith AK, Albus KE, Alaghband Rad J, Rapoport JL. Childhood‐onset schizophrenia. A double‐blind clozapine‐haloperidol comparison. Archives of General Psychiatry 1996;53(12):1090‐7. [MEDLINE: ; 8956674] - PubMed
-
- Kumra S, Jacobsen LK, Rapoport JL. Childhood‐onset schizophrenia ‐ a double‐blind clozapine trial. 149th Annual Meeting of the American Psychiatric Association; 1996 Oct 4 ‐ May 9; New York, USA. 1996. [MEDLINE: ]
Shaw 2006 {published data only}
-
- Shaw P, Sporn A, Gogtay N, Overman GP, Greenstein D, Gochman P, Tossell JW, Lenane M, Rapoport JL. Childhood‐onset schizophrenia: a double‐blind, randomized clozapine‐olanzapine comparison. Archives of General Psychiatry 2006;63(7):721‐30. [MEDLINE: ] - PubMed
Xiong 2004 {published data only}
-
- Xiong Y. Comparison study of childhood schizophrenia treated with risperidone and chlorpromazine. Guizhou Medical Journal 2004;28(8):697‐698. [114314]
Yao 2003 {published data only}
-
- Yao H. A study of risperidone in the treatment of child schizophrenia. Journal of Clinical Psychological Medicine 2003;7468(2):801. [MEDI0307]
References to studies excluded from this review
Claghorn 1972 {published data only}
-
- Claghorn JL. A double‐blind comparison of haloperidol (haldol) and thioridazine (mellaril) in outpatient children. Current Therapeutic Research 1972;14(12):785‐9. [MEDLINE: ; PsycINFO 51‐01570] - PubMed
Fish 1966 {published data only}
-
- Fish B, Shapiro T, Campbell M. Long‐term prognosis and the response of schizophrenic children to drug therapy: a controlled study of trifluoperazine. American Journal of Psychiatry 1966;123(1):32‐9. [MEDLINE: ] - PubMed
Frazier 1994 {published data only}
-
- Frazier JA, Gordon CT, McKenna K, Lenane MC, Jih D, Rapoport JL. An open trial of clozapine in 11 adolescents with childhood onset schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry 1994;33(5):658‐63. [MEDLINE: ] - PubMed
Gram 1972 {published data only}
-
- Gram LF, Rafaelsen OJ. Lithium treatment of psychotic children and adolescents. A controlled clinical trial. Acta Psychiatrica Scandinavica 1972;48(3):253‐60. [MEDLINE: ] - PubMed
Lewis 1972 {published data only}
-
- Lewis PJ, James NM. Haloperidol and chlorpromazine ‐ a double‐blind cross‐over trial and clinical study in children and adolescents. Australian and New Zealand Journal of Psychiatry 1973;7(1):59‐65. [MEDLINE: ] - PubMed
Liang 2003 {published data only}
-
- Liang L. A clinical trial of risperidone in treatment of childhood patients with first‐episode schizophrenia. Journal of Clinical Psychological Medicine 2003;13(1):20‐1. [MEDI0306]
McGlashan 2003 {published data only}
-
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein L, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, double‐blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. American Journal of Psychiatry 2006;163(5):790‐9. [MEDLINE: ] - PubMed
-
- McGlashan TH, Zipursky RB, Perkins DO, Addington J, Woods SW, Miller TJ, Lindborg S, Marquez E, Hawkins K, Hoffman RE. Olanzapine versus placebo treatment of the schizophrenia prodrome: One year results. Schizophrenia Research 2003;60:295. [MEDLINE: ]
Nagaraja 1977 {published data only}
-
- Nagaraja J. Clinical use of haloperidol (serenace) in child psychiatry. Child Psychiatry Quarterly 1977;10(4):14‐20. [PsycINFO 61‐04251]
Sikich 2004 {published data only}
-
- Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double‐blind, randomized, 8‐week trial. Neuropyschopharmacology 2004;29(1):133‐45. [MEDLINE: ; EMBASE: 2004025276] - PubMed
Spencer 1996 {published data only}
-
- Spencer EK, Kafantaris V, Padron G, Maria V, Rosenberg CR. Haloperidol in hospitalized schizophrenic children. In: Richardson MA, Haugland G editor(s). Use of neuroleptics in children. Washington, USA: American Psychiatric Press Inc, 1996:67‐83. [PsycINFO 95‐364037‐004]
-
- Spencer EK, Kafantaris V, Padron‐Gayol MV, Rosenberg CR, Campbell M. Haloperidol in schizophrenic children: early findings from a study in progress. Psychopharmacology Bulletin 1992;28(2):183‐6. [MEDLINE: ; PsycINFO 80‐02673] - PubMed
Tandon 2005 {published data only}
-
- Tandon R, Sitholey P, Katiyar M, Kumar A. Short term tolerability of risperidone in children and adolescents with acute and transient psychotic disorders. XIII World Congress of Psychiatry; 2005 10‐15th Sept; Cairo, Egypt. 2005. [P13.P250]
van Nimwegen 2006 {published data only}
-
- Nimwegen L, Haan L. Early withdrawal in a double‐blind randomized clinical trial with olanzapine and risperidone performed in adolescents with first psychosis. Psychopathology 2006;39(3):158. [MEDLINE: ; EMBASE 160407] - PubMed
References to studies awaiting assessment
Magnuson 2001 {published data only}
-
- Magnuson WG. Childhood onset psychotic disorders: characterization and treatment with atypical neuroleptics / Treatment of childhood onset psychotic disorders with olanzapine or clozapine. National Institutes of Health 2001. [National Institutes of Health (Study ID number) 97‐M‐0126; National Institutes of Health (NLM Identifier) NCT00001656]
Additional references
AACAP 2001
-
- Mclellan J, Werry J, Bernet W, Arnold V, Beitchman J, Benson R, Buckstein O, Kinlan J, McClellan, Rue D, Shaw J. Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(7 Supplement):4s‐23s. - PubMed
Altman 1996
Alvir 1993
-
- Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine induced agranulocytosis.. New England Journal of Medicine 1993;329:162‐167. - PubMed
Andreasen 1983
-
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City: The University of Iowa 1983.
Andreasen 1984
-
- Andreasen NC. The Scale for the Assessment of Positive Symptoms, (SAPS). Iowa City: The University of Iowa 1984.
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 3rd edition (DSM‐III). Washington DC: American Psychiatric Association, 1980.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV). Washington, DC: American Psychiatric Association, 1994.
Asarnow 1988
-
- Asarnow JR, Ben‐Meir S. Children with schizophrenia spectrum and depressive disorders: a comparative study of premorbid adjustment, onset pattern and severity of impairment. Journal of Child Psychology and Psychiatry 1988;29:477‐88. - PubMed
Asarnow 2004
-
- Asarnow Rosenbaum J, Tompson MC, McGrath EP. Annotation: Childhood‐onset schizophrenia: clinical and treatment issues. Journal of Child Psychology and Psychiatry 2004;45(2):180‐194. - PubMed
Baldessarini 1995
-
- Baldessarini RJ, Teicher M. Dosing of antipsychotic agents in paediatric populations. Journal of Child and Adolescent Psychopharmacology 1995;5(1):1‐4.
Beitchman 1985
-
- Beitchman JH. Childhood schizophrenia: a review and comparison with adult‐onset schizophrenia. Psychiatric Clinics of North America 1985;8:793‐814. - PubMed
Bland 1997
Bleuler 1911
-
- Bleuler E. Dementia praecox oder die gruppe der schizophrenien. Leipzig: Deuticke, 1911. - PubMed
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Bunney 1963
-
- Bunney WEJ, Hamburg DA. Methods for reliable longitudinal observation of behavior. Archives of General Psychiatry 1963;9:280‐294. - PubMed
Burd 1987
-
- Burd L, Fischer W, Kerbeshian J. A prevalence study of pervasive developmental disorders in North Dakota. Journal of the American Academy of Child and Adolescent Psychiatry 1987;26(5):700‐3. - PubMed
Calderoni 2001
-
- Calderoni D, Wudarsky M, Bhangoo R, Dell ML, Nicolson R, Hamburger S, Gochman P, et al. Differentating childhood‐onset schizophrenia from psychotic mood disorders. Journal of the American Academy of Child and Adolescent Psychiatry October 2001;40(10):1191‐7. - PubMed
Chalmers 1983
-
- Chalmers TC, Celano P, Sacks HS, Smith H. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309:1358‐61. - PubMed
Clark 1988
-
- Clark A F, Lewis S W. Practitioner review: Treatment of schizophrenia in childhood and adolescence. Journal of Child Psychology and Psychiatry 1988;39(8):1071‐81. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data: Abstracts of 8th International Cochrane Colloquium. 2000 Oct 25‐28th; Cape Town, South Africa.
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Garralda 1984
-
- Garralda M. Hallucinations in children with conduct and emotional disorders; I. The clinical phenomena. Psychological Medicine 1984;14:589‐96. - PubMed
Gillberg 1984
-
- Gillberg C. Infantile autism and other childhood psychoses in a sweedish urban region. Epidemiological aspects. Journal of Child Psychology and Psychiatry 1984;25:35‐43. - PubMed
Gillberg 1987
-
- Gillberg C, Steffenburg S. Outcome and prognostic factors in infantile autism and similar conditions: a population‐based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders 1987;17:273‐87. - PubMed
Gillberg 2001
-
- Gillberg C. Epidemiology of early onset schizophrenia. In: Remschmidt H editor(s). Schizophrenia in children and adolescents. Ist. Cambridge: Cambridge University Press, 2001:43‐54.
Green 1992
-
- Green W, Padron‐Gayol M, Hardesty A, Bassiri M. Schizophrenia with childhood onset: a phenomenological study of 38 cases. Journal of the American Academy of Child and Adolescent Psychiatry 1992;35:968‐76. - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy U. ECDEU assessment manual for psychopharmacology. Revised. Rockville: National Institute of Mental Health, 1976.
Hazell 1995
Hazell 2001
Higgins 2003
Higgins 2005
-
- Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: Higgins JPT, Green S editor(s). The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd., 2005.
Hollis 1995
-
- Hollis C. Child and adolescent schizophrenia (juvenile onset) schizophrenia: a case control study of premorbid developmental impairments. British Journal of Psychiatry 1995;166:489‐95. - PubMed
Hollis 2000
-
- Hollis C. Adolescent schizophrenia. Advances in Psychiatric Treatment 2000;6:83‐92.
Hollis 2001
-
- Hollis C. Diagnosis and differential diagnosis. In: Remschmidt H editor(s). Schizophrenia in children and adolescents. 1st Edition. Cambridge: Cambridge University Press, 2001.
Jacobsen 1998
-
- Jacobsen LK, Rapoport JL. Research update: Childhood‐onset schizophrenia: Implications of clinical and neurobiological research. Journal of Child Psychology and Psychiatry 1998;39(1):101‐13. - PubMed
Jadad 1996
-
- Jadad A, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Kane 1988
-
- Kane J, Honigfield G, Singer J, Meltzer H. Clozapine for treatment‐resistant schizophrenia: a double‐blind comparison with chlorpromazine.. Archives of General Psychiatry 1988;45:789‐796. - PubMed
Kanner 1943
-
- Kanner L. Autistic disturbances of affective contact. Nervous child 1943;2:217‐50. - PubMed
Kanner 1949
-
- Kanner L. Problems of nosology and psychodynamics of early infantile autism. American Journal of Orthopsychiatry 1949;19:416‐26. - PubMed
Kay 1987
-
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261‐76. - PubMed
Kolvin 1971
-
- Kolvin I. Studies in the childhood psychoses: I. Diagnostic criteria and classification. British Journal of Psychiatry 1971;118:381‐84. - PubMed
Kraeplin 1919
-
- Kraeplin E. Dementia praecox and paraphrenia (trans RM Barclay). Edinburgh: Livingstone, 1919.
Kumra 1998
-
- Kumra S. Children and adolescents with psychotic disorders. Child Psychopharmacology. Washington DC: American Psychiatric Press, 1998.
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
McKenna 1994
-
- Mckenna K, Gordon CT, Lenane M, Kaysan D, Fahey K, Rapoport J. Looking for childhood‐onset schizophrenia: The first 71 cases screeened. Journal of the American Academy of Child and Adolescent Psychiatry June 1994;33(5):636‐44. - PubMed
Moher 2001
-
- Moher D, Schultz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomised trials. Lancet 2001;357:1191‐4. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐389. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
Parry‐Jones 2001
-
- Parry‐Jones WL. Childhood psychosis and schizophrenia: a historical review. In: Remschmidt H editor(s). Schizophrenia in children and adolescents. Ist. Cambridge: Cambridge University Press, 2001:1‐23.
Remschmidt 1995
-
- Remschmidt H, Schulz E. Psychopharmacology of depressive states in childhood and adolescence. The depressed child and adolescent : Developmental and clinical perspectives. Cambridge: Cambridge University Press, 1995:253‐79.
Riddle 2001
-
- Riddle MA, Kaselic EA, Frosch E. Paediatric psychopharmocology. Journal of Child Psychology and Psychiatry 2001;42(1):73‐90. - PubMed
Russell 1994
-
- Russell AT. The clinical presentation of childhood‐onset schizophrenia. Schizophrenia Bulletin 1994;20:631‐46. - PubMed
Rutter 1967
-
- Rutter M, Greenfield D, Lockyer L. A five to fifteen year study of infantile psychosis. II. Social behavioural outcome. British Journal of Psychiatry 1967;113:1183‐99. - PubMed
Rutter 1972
-
- Rutter M. Childhood schizophrenia reconsidered. Journal of Autism & Childhood Schizophrenia 1972;2:315‐37. - PubMed
Schaffer 1983
-
- Schaffer D, Gould MS, Brasic J. A children's global assessment scale (CGAS). Archives of General Psychiatry 1983;40:1228‐1231. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Simpson 1970
-
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica AActa Psychiatrica Scandinavica 1970;Suppl 212:11‐19. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. - PubMed
Usiskin 1999
-
- Usiskin SI, Nicolson R, Krasnewich DM, Wenliang Y, Lenane M, Wudarsky M, Hamburger S, Rapoport J. Velocardiofacial syndrome in childhood‐onset schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(12):1536‐43. - PubMed
Volkmar 2001
-
- Volkmar FR. Childhood schizophrenia: developmental aspects. In: Remschmidt H editor(s). Schizophrenia in children and adolescents. 1st Edition. Cambridge: Cambridge University Press, 2001.
Waraich 2002
Watkins 1988
-
- Watkins JM, Asarnow RF, Tanguay P. Symptom development in childhood onset schizophrenia. Journal of Child Psychiatry and Psychology 1988;29:865‐978. - PubMed
Werry 1992
-
- Werry JS. Child and adolescent (early onset) schizophrenia: a review in light of DSM‐IIIR. Journal of Autism and Developmental Disorders 1992;22:601‐24. - PubMed
WHO 1978
-
- World Health Organisation. International Classification of Diseases (9th Revision) of Mental and Behavioural Disorders. Geneva: WHO, 1978.
WHO 1992
-
- World Health Organization. The ICD‐10 classification of mental and behavioural disorders. Clinical descriptions and diagnostic guidelines. Geneva: WHO, 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

