Vaccines for preventing smallpox
- PMID: 17636779
- PMCID: PMC6532594
- DOI: 10.1002/14651858.CD004913.pub2
Vaccines for preventing smallpox
Abstract
Background: Smallpox was eradicated by 1980, but its possible use as a bioweapon has rekindled interest in the development of protective vaccines. Therefore, stockpiled calf lymph-derived vaccines and recently developed cell-cultured vaccines have been investigated to contribute information to smallpox emergency response plans, while newer (non-replication competent) vaccines are developed.
Objectives: To assess the effects of smallpox vaccines in preventing the disease, in inducing immunity, and in regard to adverse events.
Search strategy: In December 2006, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2006, Issue 4), MEDLINE, EMBASE, LILACS, and Current Controlled Trials, and handsearched Index Medicus. We also searched three databases of vaccine safety in December 2005.
Selection criteria: Randomized controlled trials of smallpox vaccines versus placebo, other smallpox or non-smallpox vaccine, no intervention, or different dose of the same vaccine in people receiving smallpox vaccination irrespective of age.
Data collection and analysis: Both authors independently assessed trial quality and extracted data. We combined dichotomous data using relative risk with a random-effects model.
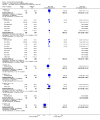
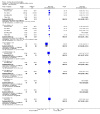
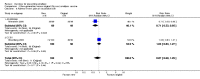
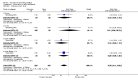
Main results: Ten trials involving 2412 participants were included. The vaccines investigated were calf-lymph derived first-generation vaccines (Dryvax, APVS, Lancy-vaxina, Lister), and cell-cultured second-generation vaccines (ACAM, CCSV). Vaccines were investigated in different dilutions. All undiluted vaccines induced a reaction in 95% of people vaccinated in terms of pustule and immunogenicity. Also 1:10 dilutions were fully efficient when the starting concentration was defined. Serious adverse events were reported in 1% to 2% of the volunteers. Fever was observed in 11% to 22% of participants, and headache in roughly half of the participants. Fever was less frequent when new vaccines were administered, but rates of headache were similar in new and old vaccines.
Authors' conclusions: The evidence shows that stockpiled vaccines have maintained their immunogenicity and new cell-cultured vaccines are similar to stockpiled vaccines in terms of vaccination success rate and immunogenicity. First- and second-generation vaccines diluted to at least 1:10 are as effective as undiluted vaccine in terms of clinical success rate and immunogenicity. Dilution did not reduce the frequency of adverse events. Success rate and immunogenicity were similar in naive and previously vaccinated persons, but there were fewer adverse events in previously vaccinated persons. The rate of adverse events found in this review reveals the need for further development and improvement of smallpox vaccines.
Conflict of interest statement
None known.
Figures













Update of
References
References to studies included in this review
Artenstein 2005 {published data only}
-
- Artenstein AW, Johnson C, Marbury TC, Morrison D, Blum PS, Kemp T, et al. A novel cell culture‐derived smallpox vaccine in vaccinia‐naive adults. Vaccine 2005;23(25):3301‐9. - PubMed
Frey 2002a {published data only}
-
- Belshe RB, Newman FK, Frey SE, Couch RB, Treanor JJ, Tacket CO, et al. Dose‐dependent neutralizing‐antibody responses to vaccinia. Journal of Infectious Diseases 2004;189(3):493‐7. - PubMed
-
- Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, Newman FK, et al. National Institute of Allergy and Infectious Diseases Smallpox Vaccine Study Group. Clinical responses to undiluted and diluted smallpox vaccine. New England Journal of Medicine 2002;346(17):1265‐74. - PubMed
Frey 2002b {published data only}
-
- Frey SE, Newman FK, Cruz J, Shelton WB, Tennant JM, Polach T, et al. Dose‐related effects of smallpox vaccine. New England Journal of Medicine 2002;346(17):1275‐80. - PubMed
Frey 2003 {published data only}
-
- Frey SE, Newman FK, Yan L, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. JAMA 2003;289(24):3294‐9. - PubMed
Greenberg 2005 {published data only}
-
- Greenberg RN, Kennedy JS, Clanton DJ, Plummer EA, Hague L, Cruz J, et al. Safety and immunogenicity of new cell‐cultured smallpox vaccine compared with calf‐lymph derived vaccine: a blind, single‐centre, randomised controlled trial. Lancet 2005;365(9457):398‐409. - PubMed
Hsieh 2006 {published data only}
-
- Hsieh SM, Chen SY, Sheu GC, Hung MN, Chou WH, Chang SC, et al. Clinical and immunological responses to undiluted and diluted smallpox vaccine with vaccinia virus of Lister strain. Vaccine 2006;24(4):510‐5. - PubMed
Kim 2005 {published data only}
-
- Kim SH, Yeo SG, Jang HC, Park WB, Lee CS, Lee KD, et al. Clinical responses to smallpox vaccine in vaccinia‐naive adults and previously vaccinated populations: undiluted and diluted Lancy‐Vaxina vaccine in a single‐blind, randomized prospective trial. Journal of Infectious Diseases 2005;192(6):1066‐70. - PubMed
Rock 2004 {published data only}
-
- Rock MT, Yoder SM, Talbot TR, Edwards KM, Crowe Jr. JE. Adverse events after smallpox immunizations are associated with alterations in systemic cytokine levels. Journal of Infectious Diseases 2004;189(8):1401‐10. - PubMed
Talbot 2004 {published data only}
-
- Talbot RT, Stapleton JT, Brady RC, Winokur PL, Bernstein DI, Germanson T, et al. Vaccination success rate and reaction profile with diluted and undiluted smallpox vaccine. JAMA 2004;292(10):1205‐12. - PubMed
-
- Talbot TR, Bredenberg HK, Smith M, LaFleur BJ, Boyd A, Edwards KM. Focal and generalized folliculitis following smallpox vaccination among vaccinia‐naive recipients. JAMA 2003;289(24):3290‐4. - PubMed
Weltzin 2003 {published data only}
-
- Weltzin R, Liu J, Pugachev KV, Myers GA, Coughlin B, Blum PS, et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nature Medicine 2003;9(9):1125‐30. - PubMed
References to studies excluded from this review
Agafonov 1977 {published data only}
-
- Agafonov VI, Vorob'ev AA, Gapochko KG, Marennikova SS, Patrikeev GT. Comparative assessment of the means and methods of immunizing humans against smallpox. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1977;6:31‐5. - PubMed
Andzhaparidze 1980 {published data only}
-
- Andzhaparidze OG, Unanov SS, Chernos VI, Strelkov VV, Antonova TP. Moscow Research Institute of Viral Preparations tissue‐culture smallpox vaccine in a coded control experiments of adult revaccination by scarification. Voprosy Virusologii 1980;4:443‐6. - PubMed
Banerji 1972 {published data only}
-
- Banerji SC, Sharma KL, Srivastava BC, Gupta RD. A comparative study of smallpox vaccination techniques under field conditions. Indian Journal of Medical Research 1972;60(5):772‐7. - PubMed
Benenson 1977 {published data only}
-
- Benenson AS, Cherry JD, McIntosh K, Connor JD, Alling DW, Nakano J, et al. Clinical and serologic study of four smallpox vaccines comparing variations of dose and route of administration. Basic study and laboratory standardization. Journal of Infectious Diseases 1977;135(1):135‐44. - PubMed
Chernos 1977 {published data only}
-
- Chernos VI, Unanov SS, Antonova TP, Nemtsov IM, Andzhaparidze OG. Study of the properties of a tissue smallpox vaccine manufactured by the Moscow Research Institute of Viral Preparations. Voprosy Virusologii 1977;1:71‐6. - PubMed
Chernos 1990 {published data only}
-
- Chernos VI, Cheliapov NV, Antonova TP, Rakhilina LE, Unanov SS, Al'tshtein AD, et al. Verification of the safety, inoculability, reactogenicity and antigenic properties of a live recombinant smallpox‐hepatitis B vaccine in an experiment in volunteers. Voprosy Virusologii 1990;35(2):132‐5. - PubMed
Cherry 1977 {published data only}
-
- Cherry JD, McIntosh K, Connor JD, Benenson AS, Alling DW, Rolfe UT, et al. Clinical and serologic study of four smallpox vaccines comparing variations of dose and route of administration. Primary percutaneous vaccination. Journal of Infectious Diseases 1977;135(1):145‐54. - PubMed
Christensen 1966 {published data only}
Denisov 1975 {published data only}
-
- Denisov GM, Kossova ET, Rybalko DI. Immunological efficacy of smallpox vaccination in children with relative contraindications to vaccination. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1975;1:68‐72. - PubMed
Evgrafov 1978 {published data only}
-
- Evgrafov VA, Kossova ET, Piskareva NA, Leonov VM, Khliabich GN. Study of the multiple puncture method in smallpox vaccination of children. Zhurnal Mikrobiologii, Epidemiologii, i Imunobiologii 1978;1:51‐3. - PubMed
Galasso 1977 {published data only}
-
- Galasso GJ, Mattheis MJ, Cherry JD, Connor JD, McIntosh K, Benenson AS. Clinical and serologic study of four smallpox vaccines comparing variations of dose and route of administration. Summary. Journal of Infectious Diseases 1977;135(1):183‐6. - PubMed
Gateff 1973 {published data only}
-
- Gateff C, Relyveld EH, Gonidec G, Vincent J, Labusquiere R, McBean M, et al. Study of a new pentavalent vaccine combination. Annales de Microbiologie 1973;124(3):387‐409. - PubMed
Herrero 1969 {published data only}
-
- Herrero E, Mutzenbecher H. Smallpox vaccine from cell cultures. Clinical trials and comparison with dermovaccine. Zentralblatt Fur Bakteriologie, Parasitenkunde, Infektionskrankheiten Und Hygiene ‐ 1 ‐ Abt ‐ Medizinisch‐Hygienische Bakteriologie, Virusforschung Und Parasitologie ‐ Originale 1969;209(4):415‐22. - PubMed
Huerta 2006 {published data only}
Karchmer 1971 {published data only}
-
- Karchmer AW, Friedman JP, Casey HL, Shope TC, Riker JB, Kappelman MM, et al. Simultaneous administration of live virus vaccines. Measles, mumps, poliomyelitis, and smallpox. American Journal of Diseases of Children 1971;121(5):382‐8. - PubMed
Kennedy 2004 {published data only}
-
- Kennedy JS, Frey SE, Yan L, Rothman AL, Cruz J, Newman FK, et al. Induction of human T cell‐mediated immune responses after primary and secondary smallpox vaccination. Journal of Infectious Diseases 2004;190(7):1286‐94. - PubMed
Khliabich 1978 {published data only}
-
- Khliabich GN, Sumarokov AA, Ozeretskovskii NA, Karinskaia GA, Gurvich EB. Comparative study of smallpox vaccines from strains B‐51, EM‐63 and L‐IVP in a controlled epidemiologic trial. I. Reactogenic characteristics of the smallpox vaccines. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1978;8:20‐6. - PubMed
Lelong 1952 {published data only}
-
- Lelong M, Ramon J. Smallpox vaccination trials using a formalin vaccine. Presse Médicale 1952;60(13):265‐6. - PubMed
Lev 1972 {published data only}
-
- Lev MI, Sergeichik II, Kulikov IA. Comparative evaluation of the needleless, syringe and scarification methods of vaccination. Voenno‐Meditsinskii Zhurnal 1972;9:955‐7. - PubMed
Lin 1965 {published data only}
Marennikova 1966 {published data only}
-
- Marennikova SS, Tashpulatov GM. Comparative study of the inoculability, reactogenicity and antigenic activity of smallpox vaccines prepared from various strains (preliminary report). Voprosy Virusologii 1966;11(3):266‐72. - PubMed
Marennikova 1977 {published data only}
-
- Marennikova SS, Matsevich GR, Sokolova AF, Shul'ga LG, Manenkova GM. Immunological characteristics of the 2‐stage method of smallpox vaccination. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1977;5:105‐9. - PubMed
Martin du Pan 1966 {published data only}
-
- Martin du Pan R. Should one vaccinate against smallpox by scarification or multipuncture? Comparative study of 2 smallpox vaccines administered in 2 different ways. Pédiatrie 1966;21(3):299‐309. - PubMed
Mel'nikov 2005 {published data only}
-
- Mel'nikov SA, Podkuiko VN, Khamitov RA, Maksimov VA, Markov VI, Vorob'ev AA, et al. Clinical studies of vaccine TEOVac under the conditions of remote revaccination. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2005;6:29‐33. - PubMed
Meyer 1964 {published data only}
Mihailescu 1988 {published data only}
-
- Mihailescu R, Velea V, Chirescu N, Topciu V, Doroiu N. Immunogenic capacity of a fractionated lyophilized smallpox vaccine. Revista de Igiena, Bacteriologie, Virusologie, Parazitologie, Epidemiologie, Pneumoftiziologie 1988;33(1):35‐9. - PubMed
Millar 1969 {published data only}
Mobest 1974 {published data only}
-
- Mobest H. Analysis of the immunogenic effect of liquid and freeze‐dried smallpox vaccine (as demonstrated in more than 20,000 persons vaccinated three times). Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Erste Abteilung Originale. Reihe A: Medizinische Mikrobiologie und Parasitologie 1974;227(1‐4):263‐81. - PubMed
Murruzzu 1967 {published data only}
-
- Murruzzu I. Comparison of the 3 types of smallpox vaccines. (Ist. Sier. Milanese, Ist. Sier. Sclavo and Ist Sier. Berna). Giornale di Batteriologia, Virologia ed Immunologia 1967;60(1):17‐21. - PubMed
Nath 1970 {published data only}
-
- Nath LM, Rao YS, Rao TM. A comparative study of the rotary lancet and bifurcate needle techniques of smallpox vaccination. Indian Journal of Medical Research 1970;58(3):388‐93. - PubMed
Neff 1969 {published data only}
Nyerges 1968 {published data only}
-
- Nyerges G, Hollos I, Losonczy G. Efficiency of smallpox revaccination repeated at three‐year intervals. II. Comparative study of the international reference strain (Elstree) and the Hungarian vaccine strain (Budapest). Acta Microbiologica Academiae Scientiarum Hungaricae 1968;15(3):187‐97. - PubMed
Nyerges 1972 {published data only}
-
- Nyerges G, Csukas M. Immunogenicity of smallpox vaccines prepared from strains of different reactogenicity. Acta Microbiologica Academiae Scientiarum Hungaricae 1972;19(2):103‐9. - PubMed
Nyerges 1973 {published data only}
Pattanayak 1970 {published data only}
Petersen 1966 {published data only}
-
- Petersen ES Norby G. A comparison between two techniques of smallpox vaccination. Danish Medical Bulletin 1966;13(6):168‐9. - PubMed
Podkuiko 1993 {published data only}
-
- Podkuiko VN, Vorob'ev AA, Krasnianskii VP, Patrikeev GT, Mikhailov VV, Dorokhina TV, et al. Peroral immunization‐‐a method for enhancing the safety of the recombinant vector (the vaccinia virus). Vestnik Rossiiskoi Akademii Meditsinskikh Nauk/Rossiiskaia Akademiia Meditsinskikh Nauk 1993;2:39‐44. - PubMed
Roberto 1969 {published data only}
Sergeev 2004 {published data only}
-
- Sergeev AA, Sergeev AN, Petrishchenko VA, Shishkina LN, Kochneva GV, Zhukov VA, et al. Reactogenicity, safety and immunogenicity of a recombinant bivaccine against smallpox and hepatitis B in limited clinical trials. Voprosy Virusologii 2004;49(5):22‐6. - PubMed
Sherman 1967 {published data only}
Shinozaki 1969 {published data only}
-
- Shinozaki T. Study of postvaccinal changes in blood antibodies. 4. Comparison of inoculation reactions using classic, standard and low toxic strains. Nippon Shonika Gakkai Zasshi. Acta Paediatrica Japonica 1969;73(4):497‐504. - PubMed
Snell 1965 {published data only}
-
- Snell PH. A comparison of two methods of vaccination against smallpox in Nigeria. West African Medical Journal 1965;14(6):233‐5. - PubMed
Tauraso 1972 {published data only}
-
- Tauraso NM, Myers MG, Nau EV, O'Brien TC, Spindel SS, Trimmer RW. Effect of interval between inoculation of live smallpox and yellow‐fever vaccines on antigenicity in man. Journal of Infectious Diseases 1972;126(4):362‐71. - PubMed
Unanov 1966 {published data only}
-
- Unanov SS, Marennikova SS, Akatova EM, Gurvich EB, Kantsova TI, Mal'tseva NN, et al. Comparative evaluation of smallpox vaccines. Voprosy Virusologii 1966;11(5):519‐25. - PubMed
Vaughan 1972 {published data only}
-
- Vaughan JP, Lindqvist K, Brooke D, Doyle RF. Combined BCG and smallpox immunization: a preliminary report on a method using the W.H.O. bifurcated needle. East African Medical Journal 1972;49(3):207‐12. - PubMed
Vaughan 1973 {published data only}
-
- Vaughan JP, Menu JP, Lindqvist KJ, Vennema A. A trial with a mixed BCG/smallpox vaccine given intradermally. Journal of Tropical Medicine and Hygiene 1973;76(10):262‐4. - PubMed
Vollmar 2006 {published data only}
-
- Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine 2006;24(12):2065‐70. - PubMed
Waibel 2004 {published data only}
-
- Waibel KH, Ager EP, Topolski RL, Walsh DS. Randomized trial comparing vaccinia on the external surfaces of 3 conventional bandages applied to smallpox vaccination sites in primary vaccinees. Clinical Infectious Diseases 2004;39(7):1004‐7. - PubMed
Waibel 2006 {published data only}
-
- Waibel KH, Golding H, Manischewitz J, King LR, Tuchscherer M, Topolski RL, et al. Clinical and immunological comparison of smallpox vaccine administered to the outer versus the inner upper arms of vaccinia‐naive adults. Clinical Infectious Diseases 2006;42(4):e16‐20. - PubMed
Wesley 1975 {published data only}
-
- Wesley RB, Speers WC, Neff JM, Ruben FL, Lourie B. Evaluation of two kinds of smallpox vaccine: CVI‐78 and calf lymph vaccine. I. Clinical and serologic response to primary vaccination. Pediatric Research 1975;9(8):624‐8. - PubMed
References to ongoing studies
Acambis‐a {unpublished data only}
-
- Acambis. Effect of dose on safety, tolerability, and immunogenicity of ACAM2000 smallpox vaccine in adults with previous smallpox vaccination. www.clinicaltrials.gov NCT00053482 (accessed 23 February 2007). [H‐400‐003]
Acambis‐b {unpublished data only}
-
- Acambis. Effect of dose on safety, tolerability, and immunogenicity of ACAM2000 smallpox vaccine in adults without previous smallpox vaccination. www.clinicaltrials.gov NCT00053495 (accessed 23 February 2007). [H‐400‐005]
Acambis‐c {published data only}
-
- Acambis. Dose safety, tolerability, and immunogenicity of ACAM1000 smallpox vaccine in adults without previous smallpox vaccination. www.clinicaltrials.gov NCT00053508 (accessed 23 February 2007). [H‐300‐002]
Acambis‐d {published data only}
-
- Acambis. Safety, tolerability, and immune response of ACAM3000 Modified Vaccinia Ankara (MVA) smallpox vaccine in adults. www.clinicaltrials.gov NCT00079820 (accessed 23 February 2007). [H‐249‐001]
Acambis‐e {published data only}
-
- Acambis. Safety study of MVA smallpox vaccine in HIV‐positive subjects who are vaccinia naive. www.clinicaltrials.gov NCT00282581 (accessed 23 February 2007). [H‐249‐004]
Acambis‐f {published data only}
-
- Acambis. Safety study of MVA smallpox vaccine in subjects with a history of atopic dermatitis (AD). www.clinicaltrials.gov NCT003389103 (accessed 23 February 2007). [H‐249‐005]
Bavarian Nordic‐a {published data only}
-
- Bavarian Nordic. Safety, tolerability and immune response of IMVAMUNE (MVA‐BN) smallpox vaccine in HIV infected patients. www.clinicaltrials.gov NCT00189904 (accessed 23 February 2007). [POX‐MVA‐010; HHSN266200400072C]
Bavarian Nordic‐b {published data only}
-
- Bavarian Nordic. Safety, tolerability and immune response of IMVAMUNE (MVA‐BN®) smallpox vaccine in patients with atopic disorders. www.clinicaltrials.gov NCT00189917 (accessed 23 February 2007). [POX‐MVA‐007; NIH NO1‐AI‐30016]
Bavarian Nordic‐c {published data only}
-
- Bavarian Nordic. Study on safety, tolerability and immunogenicity of an MVA‐BN vaccine administered to healthy subjects. www.clinicaltrials.gov NCT00189943 (accessed 23 February 2007). [MVA‐POX‐001]
Bavarian Nordic‐d {published data only}
-
- Bavarian Nordic. Dose‐finding study to evaluate the immunogenicity of three different dose levels of the IMVAMUNE (MVA‐BN) smallpox vaccine. www.clinicaltrials.gov NCT00189956 (accessed 23 February 2007). [POX‐MVA‐004; NIH N01‐AI‐30016]
Bavarian Nordic‐e {published data only}
-
- Bavarian Nordic. Take rate, immunogenicity and safety of Elstree‐BN smallpox vaccine in healthy vaccinia‐naive subjects. www.clinicaltrials.gov NCT00189969 (accessed 23 February 2007). [POX‐ELS‐001]
Bavarian Nordic‐f {published data only}
-
- Bavarian Nordic. A randomized, double‐blind, placebo‐controlled study on immunogenicity and safety of MVA‐BN® (IMVAMUNE™) smallpox vaccine in healthy subjects. www.clinicaltrials.gov NCT00316524 (accessed 23 February 2007). [POX‐MVA‐004; DMID 05‐0128; EudraCT No. 2005‐001781‐14]
Bavarian Nordic‐g {published data only}
-
- Bavarian Nordic. Safety and immunogenicity of MVA‐BN® (IMVAMUNE) smallpox vaccine in HIV infected patients. www.clinicaltrials.gov NCT00316589 (accessed 23 February 2007). [POX‐MVA‐011]
Bavarian Nordic‐h {published data only}
-
- Bavarian Nordic. A Phase II study on immunogenicity and safety of MVA‐BN® (IMVAMUNE™) smallpox vaccine in subjects with atopic dermatitis. www.clinicaltrials.gov NCT00316602 (accessed 23 February 2007). [POX‐MVA‐008; DMID 05‐0133]
Bristol‐Myers Squibb {published data only}
-
- Bristol‐Myers Squibb. A comparative Phase I clinical study of HIVAC‐1e and smallpox (vaccinia) vaccines in previously (vaccinia) vaccinated and unvaccinated volunteers. www.clinicaltrials.gov NCT00002261 (accessed 23 February 2007). [063A; A1452‐003001]
DynPort {published data only}
-
- DynPort Vaccine Company. Study of reactogenicity, safety, immunogenicity, and pock lesion formation of a cell‐cultured smallpox vaccine compared to Dryvax®. www.clinicaltrials.gov NCT00042094 (accessed 23 February 2007). [SMPX‐001]
NIAID‐a {published data only}
-
- National Institute of Allergy and Infectious Diseases. A multicenter, randomized, placebo‐controlled, double‐blind trial to evaluate the safety and immunogenicity of a recombinant vaccinia‐HIV‐1 IIIB Env/Gag/Pol Vaccine (TBC‐3B). www.clinicaltrials.gov NCT00000767 (accessed 23 February 2007). [AVEG 014 A/B]
NIAID‐b {published data only}
-
- National Institute of Allergy and Infectious Diseases. Dryvax dilution ‐ prev vacc adults (dose response study of Dryvax vaccine against smallpox in previously vaccinated adults). www.clinicaltrials.gov NCT00032708 (accessed 23 February 2007).
NIAID‐c {published data only}
-
- National Institute of Allergy and Infectious Diseases. Vaccinia virus vaccine (APSV) in vaccinia‐naive subjects: PILOT (Safety and preliminary efficacy of various concentrations of Aventis Pasteur's smallpox vaccine, USP (APSV) in vaccinia‐naive adults). www.clinicaltrials.gov NCT00038987 (accessed 23 February 2007). [02‐009]
NIAID‐d {published data only}
-
- National Institute of Allergy and Infectious Diseases. Dryvax seropositives #2 (Expanded Trial) (dose‐response study of Dryvax vaccine against smallpox in previously vaccinated adults). www.clinicaltrials.gov NCT00050505 (accessed 23 February 2007). [02‐007]
NIAID‐e {published data only}
-
- National Institute of Allergy and Infectious Diseases. APSV in vaccinia‐naive adults. www.clinicaltrials.gov NCT00050518 (accessed 23 February 2007). [02‐054]
NIAID‐g {published data only}
-
- National Institute of Allergy and Infectious Diseases. Dressing preparations for smallpox (A comparison of dressing preparations for smallpox vaccination sites with a focus upon the risk of secondary transmission of vaccinia virus). www.clinicaltrials.gov NCT00063856 (accessed 23 February 2007). [03‐044]
NIAID‐h {published data only}
-
- National Institute of Allergy and Infectious Diseases. Human immune responses smallpox (evaluation of human immune response to smallpox vaccine (vaccinia virus). www.clinicaltrials.gov NCT00068198 (accessed 23 February 2007). [02‐067]
NIAID‐i {published data only}
-
- National Institute of Allergy and Infectious Diseases. Combination study with MVA BN and Dryvax (safety and immunogenicity of MVA‐BN in a dose response regimen followed by administration of Dryvax). www.clinicaltrials.gov NCT00082446 (accessed 23 February 2007). [02‐017; POX‐MVA‐002]
NIAID‐j {published data only}
-
- National Institute of Allergy and Infectious Diseases. ACAM 3000 MVA at Harvard Medical School (Acambis Modified Vaccinia Ankara) immunization followed by Dryvax vaccination of healthy vaccinia‐naïve adults). www.clinicaltrials.gov NCT00133575 (accessed 23 February 2007). [05‐0010]
NIAID‐k {published data only}
-
- National Institute of Allergy and Infectious Diseases. Phase II dose finding study of Acambis MVA smallpox vaccine. www.clinicaltrials.gov NCT00170651 (accessed 23 February 2007). [05‐0058; H‐249‐002]
NIAID‐l {published data only}
-
- National Institute of Allergy and Infectious Diseases. MVA post‐event: dosage level and boost study (evaluation of IMVAMUNE® smallpox vaccine with respect to safety and optimization of immune responses by administration timing, dose level administered, and subsequent boost with Dryvax® smallpox vaccine in vaccinia naive adults). www.clinicaltrials.gov NCT00437021 (accessed 23 February 2007). [06‐0012; POX‐MVA‐009]
NIAID‐m {published data only}
-
- National Institute of Allergy and Infectious Diseases. Phase I trial of smallpox vaccine (A Phase I/II clinical trial of Modified Vaccinia Virus Ankara (MVA) to evaluate its safety, dosing schedule, immunogenicity and protective efficacy against Dryvax challenge in vaccinia‐naive individuals). www.clinicaltrials.gov NCT00046397 (accessed 23 February 2007). [020316; 02‐I‐0316]
NIAID‐n {published data only}
-
- National Institutes of Health Clinical Center. Phase I/II trial of modified vaccinia virus Ankara (MVA) vaccine against smallpox. www.clinicaltrials.gov NCT00053742 (accessed 23 February 2007). [030087; 03‐I‐0087]
NIAID‐o {published data only}
-
- National Institutes of Health Clinical Center. Immune responses to smallpox vaccination. www.clinicaltrials.gov NCT00325975 (accessed 23 February 2007). [030090; 03‐I‐0090]
Sanofi‐Aventis {published data only}
-
- Sanofi‐Aventis. Evaluation of take and safety of a smallpox vaccine in healthy young adults. www.clinicaltrials.gov NCT00258947 (accessed 23 February 2007). [VVL04]
Seoul NU {published data only}
-
- Seoul National University Hospital. Safety and imunogenicity of CJ‐50300. www.clinicaltrials.gov NCT00336635 (accessed 23 February 2007). [CP_SPX_101]
VaxGen {published data only}
-
- VaxGen. Safety and immunogenicity of LC16m8, a modified vaccinia vaccine, in healthy, previously unvaccinated volunteers. www.clinicaltrials.gov NCT00103584 (accessed 23 February 2007). [VAX012]
Additional references
Aragon 2003
Arness 2004
-
- Arness MK, Eckart RE, Love SS, Atwood JE, Wells TS, Engler RJ, et al. Myocarditis following smallpox vaccination. American Journal of Epidemiology 2004;160(7):642‐51. - PubMed
Bicknell 2002
-
- Bicknell WJ. The case for voluntary smallpox vaccination. New England Journal of Medicine 2002;346(17):1323‐5. [MEDLINE: ] - PubMed
Brighton 2004
-
- Brighton Collaboration. Definitions & guidelines. brightoncollaboration.org/en/index/aefi.html (accessed 6 April 2004).
CDC 2006
-
- Centers for Disease Control and Prevention. Smallpox fact sheet. www.bt.cdc.gov/agent/smallpox/vaccination/facts.asp 21 February 2006 (accessed 11 January 2007).
Drexler 2003
-
- Drexler I, Staib C, Kastenmuller W, Stevanovic S, Schmidt B, Lemonnier FA, et al. Identification of vaccinia virus epitope‐specific HLA‐A*0201‐restricted T cells and comparative analysis of smallpox vaccines. Proceedings of the National Academy of Sciences of the United States of America 2003;100(1):217‐22. [MEDLINE: ] - PMC - PubMed
Fenner 1988
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization, 1988. [MEDLINE: ]
Frey 2002c
-
- Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, Newman FK, et al. National Institute of Allergy and Infectious Diseases Smallpox Vaccine Study Group. Clinical responses to undiluted and diluted smallpox vaccine. New England Journal of Medicine 2002;346(17):1265‐74. - PubMed
Grabenstein 2003
-
- Grabenstein JD, Winkenwerder W Jr. US military smallpox vaccination program experience. JAMA 2003;289(24):3278‐82. - PubMed
Henderson 1999a
Henderson 1999b
-
- Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, et al. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 1999;281(22):2127‐37. [MEDLINE: ] - PubMed
Henderson 2001
-
- Henderson DA, Fenner F. Recent events and observations pertaining to smallpox virus destruction in 2002. Clinical Infectious Diseases 2001;33(7):1057‐9. [MEDLINE: ] - PubMed
Higgins 2006
-
- Higgins JPT, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Appendix 5b. www.cochrane.org/resources/ handbook/hbook.htm (accessed 1 December 2006).
Hopkins 1988
-
- Hopkins JW. The eradication of smallpox: organizational learning and innovation in international health administration. Journal of Developing Areas 1988;22(3):321‐32. [MEDLINE: ] - PubMed
HRSA 2006
-
- Health Resources and Services Adminstration. Smallpox Vaccine Injury Compensation Program: Administrative implementation. Federal Register 2006;71(100):29805‐11. - PubMed
Jüni 2001
Kenner 2006
Kretschmar 2006
Lane 1970a
-
- Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. Journal of Infectious Diseases 1970a;122(4):303‐9. [MEDLINE: ] - PubMed
Lane 1970b
-
- Lane JM, Ruben FL, Abrutyn E, Millar JD. Deaths attributable to smallpox vaccination, 1959 to 1966, and 1968. JAMA 1970b;212(3):441‐4. - PubMed
Lane 2003
-
- Lane MJ, Goldstein J. Evaluation of 21st‐century risks of smallpox vaccination and policy options. Annals of Internal Medicine 2003;138(6):488‐93. - PubMed
MMWR 2003
-
- Centers for Disease Control and Prevention. Smallpox vaccine adverse events monitoring and response system for the first stage of the smallpox vaccination program. MMWR 2003;52(5):88‐9, 99. [MEDLINE: ] - PubMed
MMWR 2006
-
- Casey C, Vellozzi C, Mootrey GT, Chapman LE, McCauly M, Roper MH, et al. Vaccinia Case Definition Development Working Group, Advisory Committee on Immunization Practices‐Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR 2006;55(RR‐1):1‐16. - PubMed
Neff 2002
-
- Neff JM, Lane JM, Fulginiti VA, Henderson DA. Contact vaccinia‐‐transmission of vaccinia from smallpox vaccination. JAMA 2002;288(15):1901‐5. [MEDLINE: ] - PubMed
Poland 2005a
-
- Poland GA, Grabenstein JD, Neff M. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine 2005;23(17‐18):2078‐81. - PubMed
Poland 2005b
-
- Poland GA. Smallpox vaccines: from first to second to third generation. Lancet 2005;365(9457):362‐3. - PubMed
Prentice 1989
-
- Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Statistics in Medicine 1989;8(4):431‐40. - PubMed
Review Manager 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sejvar 2005
-
- Sejvar J, Boneva R, Lane JM, Iskander J. Severe headaches following smallpox vaccination. Headache 2005;45(1):87‐8. - PubMed
WHO 1980
-
- WHO. Declaration of global eradication of smallpox. Weekly Epidemiological Record 1980;55:145‐52.
Wiser 2007
-
- Wiser I, Balicer RD, Cohen D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine 2007;25(6):976‐84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

