Cyclosporin A for primary biliary cirrhosis
- PMID: 17636804
- PMCID: PMC8890684
- DOI: 10.1002/14651858.CD005526.pub2
Cyclosporin A for primary biliary cirrhosis
Abstract
Background: Cyclosporin A has been used for patients with primary biliary cirrhosis, but the therapeutic responses in randomised clinical trials have been heterogeneous.
Objectives: To assess the beneficial and harmful effects of cyclosporin A for patients with primary biliary cirrhosis.
Search strategy: Relevant randomised clinical trials were identified by searching The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, The Chinese Biomedical Database, and LILACS, and manual searches of bibliographies to June 2006. We contacted authors of trials and the company producing cyclosporin A.
Selection criteria: Randomised clinical trials comparing cyclosporin A with placebo, no intervention, or another drug were included irrespective of blinding, language, year of publication, and publication status.
Data collection and analysis: Our primary outcomes were mortality, and mortality or liver transplantation. Dichotomous outcomes were reported as relative risk (RR) and if appropriate, Peto odds ratio with 95% confidence interval (CI). Continuous outcomes were reported as weighted mean difference (WMD) or standardised mean difference (SMD). We examined intervention effects by random-effects and fixed-effect models.
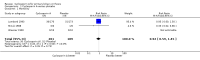
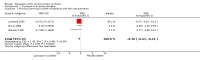
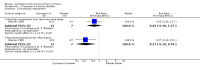
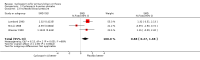
Main results: We identified three trials with 390 patients that compared cyclosporin A versus placebo. Two of them were assessed methodologically adequate with low-bias risk. Cyclosporin A did not significantly reduce mortality risk (RR 0.92, 95% CI 0.59 to 1.45), and mortality or liver transplantation (RR 0.85, 95% CI 0.60 to 1.20). Cyclosporin A significantly improved pruritus (SMD -0.38, 95% CI -0.63 to -0.14), but not fatigue. Cyclosporin A significantly reduced alanine aminotransferase (WMD -41 U/L, 95% CI -63 to -18) and increased serum albumin level (WMD 1.66 g/L, 95% CI 0.26 to 3.05). Significantly more patients experienced adverse events in the cyclosporin A group than in the placebo group, especially renal dysfunction (Peto odds ratio 5.56, 95% CI 2.52 to 12.27) and hypertension (SMD 0.88, 95% CI 0.27 to 1.48).
Authors' conclusions: We found no evidence supporting or refuting that cyclosporin A may delay death, death or liver transplantation, or progression of primary biliary cirrhosis. Cyclosporin A caused more adverse events than placebo, like renal dysfunction and hypertension. We do not recommend the use of cyclosporin A outside randomised clinical trials.
Conflict of interest statement
None known. We have no affiliations or financial contracts with companies producing the drugs examined in this review.
Figures









Update of
- doi: 10.1002/14651858.CD005526
References
References to studies included in this review
Lombard 1993 {published data only}
-
- Lombard M, Portmann B, Neuberger J, Williams R, Tygstrup N, Ranek L, et al. Cyclosporin A treatment in primary biliary cirrhosis: results of a long‐term placebo controlled trial. Gastroenterology 1993;104:519‐26. - PubMed
-
- Robson SC, Neuberger JM, Williams R. The influence of cyclosporine A therapy on sex hormone levels in pre‐ and post‐menopausal women with primary biliary cirrhosis. Journal of Hepatology 1994;21:412‐4. - PubMed
Minuk 1988 {published data only}
-
- Hanley DA, Ayer LM, Gundberg CM, Minuk GY. Parameters of calcium metabolism during a pilot study of cyclosporin A in patients with symptomatic primary biliary cirrhosis. Clinical and Investigative Medicine 1991;14(4):282‐7. - PubMed
-
- Minuk GY, Bohme CE, Burgess E, Hershfield NB, Kelly JK, Shaffer EA, et al. Pilot study of cyclosporin A in patients with symptomatic primary biliary cirrhosis. Gastroenterology 1988;95:1356‐63. - PubMed
-
- Parsons HG, Thirsk JE, Frohlich J, Dias V, Minuk GY. Effect of cyclosporin A on serum lipids in primary biliary cirrhosis patients. Clinical and Investigative Medicine 1989;12(6):386‐91. - PubMed
Wiesner 1990 {published data only}
-
- Wiesner RH, Ludwig J, Lindor KD, Jorgensen RA, Baldus WP, Homburger HA, et al. A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. New England Journal of Medicine 1990;322(20):1419‐24. - PubMed
References to studies excluded from this review
Chau 2001 {published data only}
-
- Chau TN, Quaglia A, Rolles K, Burroughs AK, Dhillon AP. Histological patterns of rejection using oral microemulsified cyclosporine and tacrolimus (FK 506) as monotherapy induction after orthotopic liver transplantation. Liver 2001;21:329‐34. - PubMed
Dmitrewski 1996 {published data only}
-
- Dmitrewski J, Hubscher SG, Mayer AD, Neuberger M. Recurrence of primary biliary cirrhosis in the liver allograft: the effect of immunosuppression. Journal of Hepatology 1996;24:253‐7. - PubMed
McMichael 1993 {published data only}
-
- McMichael J, Lieberman R, Doyle H, McCauley J, Thiel D, Thomson A. Computer‐guided concentration‐controlled trials in autoimmune disorders. Therapeutic Drug Monitoring 1993;15:510‐3. - PubMed
McMichael 1996 {published data only}
-
- McMichael J, Lieberman R, McCauley J, Irish W, Marino I, Doyle H. Computer‐guided randomized concentration‐controlled trials of tacrolimus in autoimmunity: multiple sclerosis and primary biliary cirrhosis. Therapeutic Drug Monitoring 1996;18(4):435‐7. - PubMed
Mueller 1995 {published data only}
-
- Mueller AR, Platz KP, Bechstein WO, Blumhardt G, Christe W, Hopf U, et al. The optimal immunosuppressant after liver transplantation according to diagnosis: Cyclosporine A or FK506?. Clinical Transplantation 1995;9:176‐84. - PubMed
Robert 2003 {published data only}
-
- Robert L, Carithers Jr. Primary biliary cirrhosis: specific treatment. Clinical Liver Disease 2003;7:923‐39. - PubMed
Sanchez 2003 {published data only}
-
- Sanchez EQ, Levy MF, Goldstein RM, Fasola CG, Tillery GW, Netto GJ. The changing clinical presentation of recurrent primary biliary cirrhosis after liver transplantation. Transplantation 2003;76:1583‐8. - PubMed
Slitzky 1990 {published data only}
-
- Slitzky BE, Ouellette GS, Boyer JL. Approaches to the treatment of primary biliary cirrhosis ‐ a status report. Gastroenterology International 1990;3(3):134‐9.
von Graffenried 1985 {published data only}
-
- Graffenried B, Harrison WB. Renal function in patients with autoimmune diseases treated with cyclosporine. Transplantation Proceedings 1985;17(4 Suppl 1):215‐31. - PubMed
Additional references
Christensen 1985
-
- Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, et al. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology 1985;89:1084‐91. [MEDLINE: ] - PubMed
Cohen 1984
-
- Cohen DJ, Loertscher R, Runin MF, Tilney NL, Carpenter CB, Strom TB. Cyclosporine: a new immunosuppressive agent for organ transplantation. Annals of Internal Medicine 1984;101:667‐82. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] - PubMed
Dickson 1985
-
- Dickson ER, Fleming TR, Wiesner RH, Baldus WP, Fleming CR, Ludwig J, et al. Trial of penicillamine in advanced primary biliary cirrhosis. New England Journal of Medicine 1985;312(16):1011‐5. [MEDLINE: ] - PubMed
Fregeau 1989
-
- Fregeau D, Water J, Danner D, Ansart T, Coppel R, Gershwin M. Antimitochondrial antibodies AMA of primary biliary cirrhosis PBC recognize dihydrolipoamide acyltransferase and inhibit enzyme function of the branched chain alpha ketoacid dehydrogenase complex. Faseb Journal 1989;3:A1121. [MEDLINE: ] - PubMed
Gluud 2007
-
- Gluud C, Nikolova D, Klingenberg SL, Als‐Nielsen B, D'Amico G, Davidson B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2007, Issue 2. Art. No.: LIVER.
Gong 2004
Gong 2005a
-
- Gong Y, Frederiksen SL, Gluud C. Methotrexate for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No.: CD004385. DOI: 10.1002/14651858.CD004385.pub2. - PubMed
Gong 2005b
-
- Gong Y, Gluud C. Colchicine for primary biliary cirrhosis: a systematic review of randomised clinical trials. American Journal of Gastroenterology 2005;100:1876‐85. - PubMed
Gong 2005c
Harris 1987
-
- Harris DT, Kozumbo WJ, Cerutti PA, Cerottini JC. Mechanism of cyclosporin A induced immunosuppression. Cell Immunology 1987;109:104‐14. - PubMed
Heathcote 1976
-
- Heathcote J, Ross A, Sherlock S. A prospective controlled trial of azathioprine in primary biliary cirrhosis. Gastroenterology 1976;70(5 Pt 1):656‐60. [MEDLINE: ] - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2006
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & sons, Ltd.
Hoofnagle 1986
-
- Hoofnagle JH, Davis GL, Schafer DF, Peters M, Avigan MI, Pappas SC, et al. Randomized trial of chlorambucil for primary biliary cirrhosis. Gastroenterology 1986;91(6):1327‐34. [MEDLINE: ] - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation of Technical Requiements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1997.
Invernizzi 1997
-
- Invernizzi P, Crosignani A, Battezzati PM, Covini G, De‐Valle G, Larghi A, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody‐positive and negative primary biliary cirrhosis. Hepatology 1997;25(5):1090‐5. [MEDLINE: ] - PubMed
Ioannidis 2001
James 1981
-
- James O, Macklon AF, Waston AJ. Primary biliary cirrhosis ‐ a revised clinical spectrum. Lancet 1981;1(8233):1278‐81. [MEDLINE: ] - PubMed
James 1983
-
- James SP, Hoofnagle J, Strober W, Jones EA. Primary biliary cirrhosis: a model autoimmune disease. Annals of Internal Medicine 1983;99:500‐12. - PubMed
Kaplan 1991
-
- Kaplan MM, Knox TA. Treatment of primary biliary cirrhosis with low‐dose weekly methotrexate. Gastroenterology 1991;101:1332‐8. - PubMed
Kaplan 1996
-
- Kaplan MM. Primary biliary cirrhosis. New England Journal of Medicine 1996;335(21):1570‐80. - PubMed
Kim 2000
-
- Kim WR, Lindor KD, Locke GR 3rd, Therneau TM, Homburger HA, Batts KP, et al. Epidemiology and natural history of primary biliary cirrhosis in a U.S. community. Gatroenterology 2000;119:1631‐6. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lacerda 1995
-
- Lacerda MA, Ludwig J, Dickson ER, Jorgensen RA, Lindor KD. Antimitochondrial antibody‐negative primary biliary cirrhosis. American Journal of Gastroenterology 1995;90(2):247‐9. [MEDLINE: ] - PubMed
Lindor 1995
-
- Lindor KD, Dickson ER, Jorgensen RA, Anderson ML, Wiesner RH, Gores GJ, et al. The combination of ursodeoxycholic acid and methotrexate for patients with primary biliary cirrhosis: the results of a pilot study. Hepatology 1995;22(4 Pt 1):1158‐62. [MEDLINE: ] - PubMed
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of National Cancer Institute 1959;22:719‐48. - PubMed
Mattalia 1998
-
- Mattalia A, Quaranta S, Leung PS, Bauducci M, Van‐de‐Water J, Calvo PL. Characterization of antimitochondrial antibodies in health adults. Hepatology 1998;27(3):656‐61. [MEDLINE: ] - PubMed
Mitchison 1992
-
- Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three‐year results. Journal of Hepatology 1992;15(3):336‐44. [MEDLINE: ] - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] - PubMed
Neuberger 1985
Poupon 1996
-
- Poupon RE, Huet PM, Poupon R, Bonnand AM, Nhieu JT, Zafrani ES, et al. A randomized trial comparing colchicine and ursodeoxycholic acid combination to ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1996;24(5):1098‐103. [MEDLINE: ] - PubMed
Prince 2003
-
- Prince MI, James OFW. The epidemiology of primary biliary cirrhosis. Clinics in Liver Disease 2003;7:795‐819. - PubMed
RevMan 2003 [Computer program]
-
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Routhier 1980
-
- Routhier G, Epstein O, Janossy G, Thomas HC, Sherlock S. Effects of cyclosporin A on suppressor and induceer T lymphocytes in primary biliary cirrhosis. Lancet 1980;2:1223‐6. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Scheuer 1967
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] - PubMed
Tugwell 1990
-
- Tugwell P, Bombardier C, Gent M, Bennett KJ, Bensen WG, Carette S, et al. Low dose cyclosporin versus placebo in patients with rheumatoid arthritis. Lancet 1990;335:1051‐5. - PubMed
Turchany 1997
-
- Turchany JM, Uibo R, Kivik T, Van‐de‐Water J, Prindiville T, Coppel RL, et al. A study of antimitochondrial antibodies in a random population in Estonia. American Journal of Gastroenterology 1997;92(1):124‐6. [MEDLINE: ] - PubMed
Verma 1999
-
- Verma A, Jazrawi RP, Ahmed HA, Northfield TC. Prescribing habits in primary biliary cirrhosis: a national survey. European Journal of Gastroenterology and Hepatology 1999;11(8):817‐20. [MEDLINE: ] - PubMed
Vuoristo 1995
-
- Vuoristo M, Farkkila M, Karvonen AL, Leino R, Lehtola J, Makinen J, et al. A placebo‐controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid [see comments]. Gastroenterology 1995;108(5):1470‐8. [MEDLINE: ] - PubMed
Warnes 1987
-
- Warnes TW, Smith A, Lee FI, Haboubi NY, Johnson PJ, Hunt L. A controlled trial of colchicine in primary biliary cirrhosis: trial design and preliminary report. Jounal of Hepatology 1987;5(1):1‐7. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

