Top-down and bottom-up mass spectrometric characterization of human myoglobin-centered free radicals induced by oxidative damage
- PMID: 17637042
- PMCID: PMC2376835
- DOI: 10.1021/ac070935z
Top-down and bottom-up mass spectrometric characterization of human myoglobin-centered free radicals induced by oxidative damage
Abstract
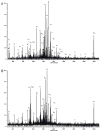
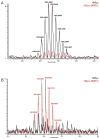
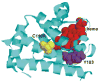
In an effort to determine the utility of top-down mass spectrometric methodologies for the characterization of protein radical adducts, top-down approaches were investigated and compared to the traditional bottom-up approaches. Specifically, the nature of the radicals on human myoglobin induced by the addition of hydrogen peroxide and captured by the spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was investigated. The most abundant ion observed in the electrospray mass spectrum of this reaction mixture corresponds in mass to the human myoglobin plus one DMPO molecule. In addition, a second ion of lower abundance is observed, which corresponds to a second DMPO molecule being trapped on myoglobin. Top-down analyses using Fourier transform ion cyclotron resonance mass spectrometry can be used to characterize proteins and, thus, were performed on several different charge-state ions of both the native and the mono-DMPO nitrone adduct of human myoglobin. Data produced from the top-down analyses are very complex yet information rich. In the case of DMPO-modified human myoglobin, the top-down data localized the DMPO spin trap to residues 97-110 of the myoglobin. The observation of the y43+5 fragment ion arising from C-terminal cleavage to the cysteine-110 residue in the MS/MS spectrum of DMPO-modified myoglobin and not in the unmodified myoglobin implicates a change to this residue, specifically, DMPO adduction. On the other hand, using the traditional bottom-up approach of peptide mapping and MS sequencing methodologies, two DMPO radical adducts on human myoglobin were identified, Cys-110 and Tyr-103. The bottom-up approach is more proven and robust than the top-down methodologies. Nonetheless, the bottom-up and top-down approaches to protein characterization are complementary rather than competitive approaches with each having its own utility.
Figures






Similar articles
-
Electron transfer between a tyrosyl radical and a cysteine residue in hemoproteins: spin trapping analysis.J Am Chem Soc. 2007 Nov 7;129(44):13493-501. doi: 10.1021/ja073349w. Epub 2007 Oct 16. J Am Chem Soc. 2007. PMID: 17939657
-
Reaction of human myoglobin and H2O2. Electron transfer between tyrosine 103 phenoxyl radical and cysteine 110 yields a protein-thiyl radical.J Biol Chem. 2001 May 11;276(19):16540-7. doi: 10.1074/jbc.M011707200. Epub 2001 Feb 13. J Biol Chem. 2001. PMID: 11278969
-
Reaction of human myoglobin and H2O2. Involvement of a thiyl radical produced at cysteine 110.J Biol Chem. 2000 Jul 7;275(27):20391-8. doi: 10.1074/jbc.M000373200. J Biol Chem. 2000. PMID: 10779502
-
Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping.Free Radic Biol Med. 2004 May 15;36(10):1214-23. doi: 10.1016/j.freeradbiomed.2004.02.077. Free Radic Biol Med. 2004. PMID: 15110386 Review.
-
In Vivo and In Situ Detection of Macromolecular Free Radicals Using Immuno-Spin Trapping and Molecular Magnetic Resonance Imaging.Antioxid Redox Signal. 2018 May 20;28(15):1404-1415. doi: 10.1089/ars.2017.7390. Epub 2017 Dec 11. Antioxid Redox Signal. 2018. PMID: 29084431 Review.
Cited by
-
Oxidative protein labeling in mass-spectrometry-based proteomics.Anal Bioanal Chem. 2010 Aug;397(8):3441-55. doi: 10.1007/s00216-010-3471-8. Epub 2010 Feb 13. Anal Bioanal Chem. 2010. PMID: 20155254 Free PMC article. Review.
-
Identifying the site of spin trapping in proteins by a combination of liquid chromatography, ELISA, and off-line tandem mass spectrometry.Free Radic Biol Med. 2008 Mar 1;44(5):893-906. doi: 10.1016/j.freeradbiomed.2007.11.015. Epub 2007 Dec 5. Free Radic Biol Med. 2008. PMID: 18160050 Free PMC article.
-
Biotinylated analogue of the spin-trap 5,5-dimethyl-1-pyrroline-N-oxide for the detection of low-abundance protein radicals by mass spectrometry.Anal Chem. 2010 Nov 15;82(22):9155-8. doi: 10.1021/ac1023183. Epub 2010 Oct 19. Anal Chem. 2010. PMID: 20957988 Free PMC article.
-
Plant based production of myoglobin - a novel source of the muscle heme-protein.Sci Rep. 2020 Jan 22;10(1):920. doi: 10.1038/s41598-020-57565-y. Sci Rep. 2020. PMID: 31969582 Free PMC article.
-
Cu,Zn-superoxide dismutase-driven free radical modifications: copper- and carbonate radical anion-initiated protein radical chemistry.Biochem J. 2009 Jan 1;417(1):341-53. doi: 10.1042/BJ20070722. Biochem J. 2009. PMID: 18764780 Free PMC article.
References
-
- Janssen YMW, Van Houten B, Borm PJA, Mossman BT. Lab Invest. 1993;69:261–274. - PubMed
-
- Kappus H. In: Oxidative Stress. Sies H, editor. Academic Press; London: 1985. pp. 273–310.
-
- Brigelius R. In: Oxidative Stress. Sies H, editor. Academic Press; London: 1985. pp. 243–274.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

