Signals emanating from the membrane proximal region of the thrombopoietin receptor (mpl) support hematopoietic stem cell self-renewal
- PMID: 17637498
- PMCID: PMC2062518
- DOI: 10.1016/j.exphem.2007.05.010
Signals emanating from the membrane proximal region of the thrombopoietin receptor (mpl) support hematopoietic stem cell self-renewal
Abstract
Objective: Studies using thrombopoietin -/- (TPO(-/-)) or TPO receptor, mpl(-/-) mice have established a critical role for TPO/mpl signaling in hematopoietic stem cell (HSC) development. In this study, we further dissected mpl signaling in both megakaryopoiesis and HSC function, using mice bearing a truncated mpl receptor lacking the distal 60 amino acids (Delta60). This deletion removes three major signaling tyrosines on the mpl cytoplasmic domain, but retains the membrane proximal Box1 and Box2 domains required for JAK2 activation.
Materials and methods: Competitive bone marrow transplantations (BMT) and serial BMTs were performed to study HSC function. Western blot analysis was used to study TPO-stimulated signaling pathways. BM cell cultures in the presence of TPO were used to study megakaryocyte development.
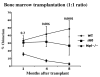
Results: In agreement with prior findings, we show that Delta60 BM cells cultured in TPO generated normal numbers of megakaryocytes, but with greatly reduced ploidy. As expected from the deletion of three signaling tyrosine residues, freshly isolated Delta60 megakaryocytes showed marked reduction in all known TPO-stimulated signaling pathways tested, including signal transducers and activators of transcription (Stat) 5, Stat3, Akt, and p42/44 mitogen-activated kinase. We found that Delta60 mice displayed normal short-term (ST-HSC) activities and marginally compromised long-term (LT-HSC) stem cell activities in primary transplantation. In addition, Delta60 mice supported HSC self-renewal for at least two serial BMTs.
Conclusion: Our data reveal a pivotal role for an unknown signal emanating from the membrane proximal region of the mpl receptor or from JAK2 itself in maintaining stem cell activity and self-renewal, in addition to its role in megakaryocytopoiesis and thrombopoiesis.
Figures


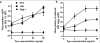




Similar articles
-
Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis.J Exp Med. 2004 Sep 6;200(5):569-80. doi: 10.1084/jem.20040762. Epub 2004 Aug 30. J Exp Med. 2004. PMID: 15337790 Free PMC article.
-
Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2.J Clin Invest. 2008 Aug;118(8):2832-44. doi: 10.1172/JCI35808. J Clin Invest. 2008. PMID: 18618018 Free PMC article.
-
Role of the distal half of the c-Mpl intracellular domain in control of platelet production by thrombopoietin in vivo.Mol Cell Biol. 2000 Jan;20(2):507-15. doi: 10.1128/MCB.20.2.507-515.2000. Mol Cell Biol. 2000. PMID: 10611229 Free PMC article.
-
Thrombopoietin and hematopoietic stem cells.Cell Cycle. 2011 May 15;10(10):1582-9. doi: 10.4161/cc.10.10.15619. Epub 2011 May 15. Cell Cycle. 2011. PMID: 21478671 Free PMC article. Review.
-
Studies of the c-Mpl thrombopoietin receptor through gene disruption and activation.Stem Cells. 1996;14 Suppl 1:124-32. doi: 10.1002/stem.5530140716. Stem Cells. 1996. PMID: 11012212 Review.
Cited by
-
Thrombopoietin mimetic stimulates bone marrow vascular and stromal niches to mitigate acute radiation syndrome.Stem Cell Res Ther. 2024 Apr 29;15(1):123. doi: 10.1186/s13287-024-03734-z. Stem Cell Res Ther. 2024. PMID: 38679747 Free PMC article.
-
Incomplete restoration of Mpl expression in the mpl-/- mouse produces partial correction of the stem cell-repopulating defect and paradoxical thrombocytosis.Blood. 2009 Feb 19;113(8):1778-85. doi: 10.1182/blood-2007-11-124859. Epub 2008 Sep 16. Blood. 2009. PMID: 18796624 Free PMC article.
-
Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes.J Clin Invest. 2017 Jun 1;127(6):2133-2147. doi: 10.1172/JCI92450. Epub 2017 May 15. J Clin Invest. 2017. PMID: 28504650 Free PMC article.
-
The thrombopoietin mimetic romiplostim leads to the complete rescue of mice exposed to lethal ionizing radiation.Sci Rep. 2018 Jul 13;8(1):10659. doi: 10.1038/s41598-018-29013-5. Sci Rep. 2018. PMID: 30006622 Free PMC article.
-
14-3-3 regulates the LNK/JAK2 pathway in mouse hematopoietic stem and progenitor cells.J Clin Invest. 2012 Jun;122(6):2079-91. doi: 10.1172/JCI59719. Epub 2012 May 1. J Clin Invest. 2012. PMID: 22546852 Free PMC article.
References
-
- Kaushansky K. Thrombopoietin: the primary regulator of platelet production. Blood. 1995;86:419–431. - PubMed
-
- Kaushansky K. Thrombopoietin: a tool for understanding thrombopoiesis. J Thromb Haemost. 2003;1:1587–1592. - PubMed
-
- Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. - PubMed
-
- Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

