The neuro-steroid, 3beta androstene 17alpha diol exhibits potent cytotoxic effects on human malignant glioma and lymphoma cells through different programmed cell death pathways
- PMID: 17637679
- PMCID: PMC2360358
- DOI: 10.1038/sj.bjc.6603894
The neuro-steroid, 3beta androstene 17alpha diol exhibits potent cytotoxic effects on human malignant glioma and lymphoma cells through different programmed cell death pathways
Abstract
The neuro-steroids 3beta-androstene-17alpha-diol (17alpha-AED), 3beta-androstene-17beta-diol (17beta-AED), 3beta-androstene-7alpha,-17beta-triol (7alpha-AET) and 3beta-androstene-7beta,-17beta-triol (7beta-AET) are metabolites of dehydroepiandrosterone and are produced in neuro-ectodermal tissue. Both epimers of androstenediols (17alpha-AED and 17beta-AED) and androstenetriols (7alpha-AET and 7beta-AET) have markedly different biological functions of their chemical analogue. We investigated the cytotoxic activity of these neuro-steroids on human T98G and U251MG glioblastoma and U937 lymphoma cells. Proliferation studies showed that 17alpha-AED is the most potent inhibitor, with an IC(50) approximately 15 microM. For T98G glioma, 90% inhibition was achieved with 25 muM of 17alpha-AED. Other neuro-steroids tested only marginally suppressed cell proliferation. Reduced cell adherence and viability could be detected after 18 h of 17alpha-AED exposure. Treatment with 17alpha-AED induced a significant level of apoptosis in U937 lymphoma cells, but not in the glioma cells. Cytopathology of 17alpha-AED-treated T98G cells revealed the presence of multiple cytoplasmic vacuoles. Acridine orange staining demonstrated the formation of acidic vesicular organelles in 17alpha-AED-treated T98G and U251MG, which was inhibited by bafilomycin A1. These findings indicate that 17alpha-AED bears the most potent cytotoxic activity of the neuro-steroids tested, and the effectiveness may depend on the number of hydroxyls and their position on the androstene molecule. These cytotoxic effects may utilize a non-apoptotic pathway in malignant glioma cells.
Figures

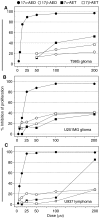
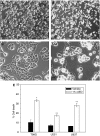


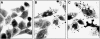

References
-
- Ben Nathan D, Padgett DA, Loria RM (1999) Androstenediol and dehydroepiandrosterone protect mice against lethal bacterial infections and lipopolysaccharide toxicity. J Med Microbiol 48: 425–431 - PubMed
-
- Boccuzzi G, Di Monaco M, Brignardello E, Leonardi L, Gatto V, Pizzini A, Gallo M (1993) Dehydroepiandrosterone antiestrogenic action through androgen receptor in MCF-7 human breast cancer cell line. Anticancer Res 13: 2267–2272 - PubMed
-
- Bursch W, Ellinger A, Gerner C, Frohwein U, Schulte-Hermann R (2000) Programmed cell death (PCD). Apoptosis, autophagic PCD, or others? Ann N Y Acad Sci 926: 1–12 - PubMed
-
- Huynh PN, Carter Jr WH, Loria RM (2000) 17 Alpha androstenediol inhibition of breast tumor cell proliferation in estrogen receptor-positive and -negative cell lines. Cancer Detect Prev 24: 435–444 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

