Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia
- PMID: 17637706
- PMCID: PMC2605394
- DOI: 10.1038/sj.jcbfm.9600527
Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia
Abstract
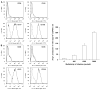
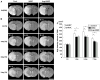
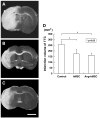
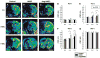
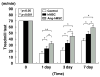
Transplantation of human mesenchymal stem cells (hMSCs) prepared from adult bone marrow has been reported to ameliorate functional deficits after cerebral artery occlusion in rats. Although several hypotheses to account for these therapeutic effects have been suggested, current thinking is that both neuroprotection and angiogenesis are primarily responsible. In this study, we compared the effects of hMSCs and angiopoietin-1 gene-modified hMSCs (Ang-hMSCs) intravenously infused into rats 6 h after permanent middle cerebral artery occlusion. Magnetic resonance imaging and histologic analyses revealed that rats receiving hMSCs or Ang-hMSCs exhibited comparable reduction in gross lesion volume as compared with the control group. Although both cell types indeed improved angiogenesis near the border of the ischemic lesions, neovascularization and regional cerebral blood flow were greater in some border areas in Ang-hMSC group. Both hMSC- and Ang-hMSC-treated rats showed greater improved functional recovery in the treadmill stress test than did control rats, but the Ang-hMSC group was greater. These results indicate the intravenous administration of genetically modified hMSCs to express angiopoietin has a similar effect on reducing lesion volume as hMSCs, but the Ang-hMSC group showed enhanced regions of increased angiogenesis at the lesion border, and modest additional improvement in functional outcome.
Figures


Similar articles
-
Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia.Exp Neurol. 2009 Mar;216(1):47-55. doi: 10.1016/j.expneurol.2008.11.010. Epub 2008 Nov 27. Exp Neurol. 2009. PMID: 19094989
-
Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia.Brain. 2006 Oct;129(Pt 10):2734-45. doi: 10.1093/brain/awl207. Epub 2006 Aug 10. Brain. 2006. PMID: 16901914 Free PMC article.
-
Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat.J Neurosci Res. 2006 Nov 15;84(7):1495-504. doi: 10.1002/jnr.21056. J Neurosci Res. 2006. PMID: 16998918 Free PMC article.
-
Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats.Brain Res. 2008 Oct 21;1236:30-8. doi: 10.1016/j.brainres.2008.07.116. Epub 2008 Aug 9. Brain Res. 2008. PMID: 18722359 Free PMC article.
-
Therapeutic Applications of Engineered Mesenchymal Stromal Cells for Enhanced Angiogenesis in Cardiac and Cerebral Ischemia.Stem Cell Rev Rep. 2024 Nov;20(8):2138-2154. doi: 10.1007/s12015-024-10787-3. Epub 2024 Sep 21. Stem Cell Rev Rep. 2024. PMID: 39305405 Free PMC article. Review.
Cited by
-
Intravenous infusion of bone marrow-derived mesenchymal stem cells improves tissue perfusion in a rat hindlimb ischemia model.Sci Rep. 2022 Oct 10;12(1):16986. doi: 10.1038/s41598-022-18485-1. Sci Rep. 2022. PMID: 36216855 Free PMC article.
-
High-mobility group box 1: an amplifier of stem and progenitor cell activity after stroke.Acta Neurochir Suppl. 2013;118:31-8. doi: 10.1007/978-3-7091-1434-6_5. Acta Neurochir Suppl. 2013. PMID: 23564100 Free PMC article. Review.
-
Engineering Stem Cells for Biomedical Applications.Adv Healthc Mater. 2016 Jan 7;5(1):10-55. doi: 10.1002/adhm.201400842. Epub 2015 Mar 13. Adv Healthc Mater. 2016. PMID: 25772134 Free PMC article. Review.
-
Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient?Regen Med. 2011 May;6(3):367-406. doi: 10.2217/rme.11.22. Regen Med. 2011. PMID: 21548741 Free PMC article.
-
Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness.Stem Cell Rev Rep. 2020 Oct;16(5):812-827. doi: 10.1007/s12015-020-10000-1. Stem Cell Rev Rep. 2020. PMID: 32671645 Free PMC article. Review.
References
-
- Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–23. - PubMed
-
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. - PubMed
-
- Carmeliet P, Collen D. Molecular analysis of blood vessel formation and disease. Am J Physiol. 1997;273:2091–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

