Increased mitochondrial DNA induces acquired docetaxel resistance in head and neck cancer cells
- PMID: 17637738
- PMCID: PMC2268644
- DOI: 10.1038/sj.onc.1210681
Increased mitochondrial DNA induces acquired docetaxel resistance in head and neck cancer cells
Abstract
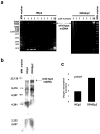
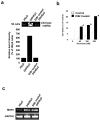
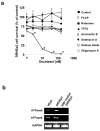
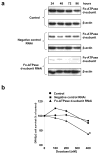
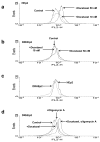
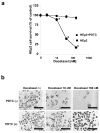
Docetaxel is one of the most effective chemotherapeutic agents against cancer; nevertheless, some patients develop resistance. Unfortunately, their causes and mechanisms remain unknown. We created docetaxel-resistant DRHEp2 from human laryngeal cancer HEp2 and investigated the roles of mitochondrial DNA (mtDNA) and reactive oxygen species (ROS) on docetaxel resistance. DRHEp2 had greatly increased mtDNA content. Reduction of mtDNA content in DRHEp2 by ethidium bromide treatment reduced the resistance. These results indicate the possible roles of mtDNA-coded enzymes in mitochondrial respiratory chain (MRC) in resistant mechanisms. Oligomycin A, an Fo-ATPase inhibitor, eliminated docetaxel resistance in DRHEp2; in contrast, inhibitors of other MRC did not. RNA interference targeted to Fo-ATPase d-subunit restored docetaxel-induced cytotoxicity to DRHEp2. These results indicate the roles of Fo-ATPase for resistant mechanisms. Docetaxel induced ROS generation in HEp2 but not in DRHEp2 and antioxidant pyrrolidine dithiocarbamate eliminated docetaxel-induced cytotoxicity, suggesting roles of ROS in docetaxel-induced cell death. Furthermore, inhibition of Fo-ATPase by Oligomycin A induced docetaxel-mediated ROS generation in DRHEp2. Taken together, DRHEp2 acquired docetaxel resistance through increasing Fo-ATPase, which led to diminish docetaxel-induced ROS generation and subsequently inhibited cell death. In conclusion, mtDNA plays an important role in developing docetaxel resistance through the reduction of ROS generation by regulating Fo-ATPase.
Figures

References
-
- Adam D, Wiegmann K, Adam-Klages S, Ruff A, Kronke M. A novel cytoplasmic domain of the p55 tumor necrosis factor receptor initiates the neutral sphingomyelinase pathway. Journal of Biological Chemistry. 1996;271:14617–14622. - PubMed
-
- Alexandre J, Batteux F, Nicco C, Chereau C, Laurent A, Guillevin L, et al. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. International Journal of Cancer. 2006;119:41–48. - PubMed
-
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. - PubMed
-
- Bissery MC, Nohynek G, Sanderink GJ, Lavelle F. Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: Preclinical experience. Anti-Cancer Drugs. 1995;6:339–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

