Simultaneous activation of complement and coagulation by MBL-associated serine protease 2
- PMID: 17637839
- PMCID: PMC1910608
- DOI: 10.1371/journal.pone.0000623
Simultaneous activation of complement and coagulation by MBL-associated serine protease 2
Abstract
The complement system is an important immune mechanism mediating both recognition and elimination of foreign bodies. The lectin pathway is one pathway of three by which the complement system is activated. The characteristic protease of this pathway is Mannan-binding lectin (MBL)-associated serine protease 2 (MASP2), which cleaves complement proteins C2 and C4. We present a novel and alternative role of MASP2 in the innate immune system. We have shown that MASP2 is capable of promoting fibrinogen turnover by cleavage of prothrombin, generating thrombin. By using a truncated active form of MASP2 as well as full-length MASP2 in complex with MBL, we have shown that the thrombin generated is active and can cleave both factor XIII and fibrinogen, forming cross-linked fibrin. To explore the biological significance of these findings we showed that fibrin was covalently bound on a bacterial surface to which MBL/MASP2 complexes were bound. These findings suggest that, as has been proposed for invertebrates, limited clotting may contribute to the innate immune response.
Conflict of interest statement
Figures



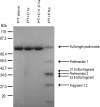
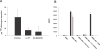
Similar articles
-
The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen.Biochim Biophys Acta. 2008 Sep;1784(9):1294-300. doi: 10.1016/j.bbapap.2008.03.020. Epub 2008 Apr 16. Biochim Biophys Acta. 2008. PMID: 18456010
-
Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot.Immunology. 2010 Apr;129(4):482-95. doi: 10.1111/j.1365-2567.2009.03200.x. Epub 2009 Dec 2. Immunology. 2010. PMID: 20002787 Free PMC article.
-
Lectin pathway of bony fish complement: identification of two homologs of the mannose-binding lectin associated with MASP2 in the common carp (Cyprinus carpio).J Immunol. 2006 Oct 15;177(8):5471-9. doi: 10.4049/jimmunol.177.8.5471. J Immunol. 2006. PMID: 17015733
-
The biological functions of MBL-associated serine proteases (MASPs).Immunobiology. 2002 Sep;205(4-5):467-75. doi: 10.1078/0171-2985-00147. Immunobiology. 2002. PMID: 12396008 Review.
-
The emerging roles of mannose-binding lectin-associated serine proteases (MASPs) in the lectin pathway of complement and beyond.Immunol Rev. 2016 Nov;274(1):98-111. doi: 10.1111/imr.12460. Immunol Rev. 2016. PMID: 27782318 Review.
Cited by
-
Complement in immune and inflammatory disorders: pathophysiological mechanisms.J Immunol. 2013 Apr 15;190(8):3831-8. doi: 10.4049/jimmunol.1203487. J Immunol. 2013. PMID: 23564577 Free PMC article. Review.
-
Complement in immune and inflammatory disorders: therapeutic interventions.J Immunol. 2013 Apr 15;190(8):3839-47. doi: 10.4049/jimmunol.1203200. J Immunol. 2013. PMID: 23564578 Free PMC article. Review.
-
Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation.PLoS One. 2012;7(4):e35690. doi: 10.1371/journal.pone.0035690. Epub 2012 Apr 20. PLoS One. 2012. PMID: 22536427 Free PMC article.
-
SARS-CoV-2: Pathogenic Mechanisms and Host Immune Response.Adv Exp Med Biol. 2021;1313:99-134. doi: 10.1007/978-3-030-67452-6_6. Adv Exp Med Biol. 2021. PMID: 34661893
-
Complement as driver of systemic inflammation and organ failure in trauma, burn, and sepsis.Semin Immunopathol. 2021 Dec;43(6):773-788. doi: 10.1007/s00281-021-00872-x. Epub 2021 Jun 30. Semin Immunopathol. 2021. PMID: 34191093 Free PMC article. Review.
References
-
- Reid KB, Porter RR. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. - PubMed
-
- Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. L-ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. 2004;279:47513–47519. - PubMed
-
- Matsushita M, Kuraya M, Hamasaki N, Tsujimura M, Shiraki H, et al. Activation of the lectin complement pathway by H-ficolin (Hakata antigen). J Immunol. 2002;168:3502–3506. - PubMed
-
- Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. - PubMed
-
- Downing MR, Butkowski RJ, Clark MM, Mann KG. Human prothrombin activation. J Biol Chem. 1975;250:8897–8906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

