The Shc-binding site of the betac subunit of the GM-CSF/IL-3/IL-5 receptors is a negative regulator of hematopoiesis
- PMID: 17638849
- PMCID: PMC2077308
- DOI: 10.1182/blood-2007-01-070391
The Shc-binding site of the betac subunit of the GM-CSF/IL-3/IL-5 receptors is a negative regulator of hematopoiesis
Abstract
Tyrosine and serine phosphorylation of the common beta chain (beta(c)) of the granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 receptors is widely viewed as a general mechanism that provides positive inputs by coupling the receptor to signaling pathways that stimulate several cellular functions. We show here that despite the known action of Tyr577 in beta(c) to recruit Shc-PI-3 kinase (PI3K) pathway members, Tyr577 plays, surprisingly, a negative regulatory role in cell function, and that this is mediated, at least in part, through the uncoupling of SH2-containing inositol 5'-phosphatase (SHIP) from beta(c). Fetal liver cells from beta(c)/beta(IL-3)(-/-) mice expressing human GM-CSF receptor alpha chain and beta(c) Tyr577Phe mutant showed enhanced colony formation and expansion of progenitor cells in response to GM-CSF. Dissection of these activities revealed that basal survival was increased, as well as cytokine-stimulated proliferation. As expected, the recruitment and activation of Shc was abolished, but interestingly, Gab-2 and Akt phosphorylation increased. Significantly, the activation of PI3K was enhanced and prolonged, accompanied by loss of SHIP activity. These results reveal a previously unrecognized negative signaling role for Tyr577 in beta(c) and demonstrate that uncoupling Shc from cytokine receptors enhances PI3K signaling as well as survival and proliferation.
Figures

 ) or wt βc (■/▨) were cultured for the indicated times in liquid media of IMDM plus 15% HI-FCS supplemented with GM-CSF at 100 ng/mL (□ and ■) or a cytokine cocktail (50 ng/mL IL-6, 100 ng/mL SCF, and 10 ng/mL G-CSF;
) or wt βc (■/▨) were cultured for the indicated times in liquid media of IMDM plus 15% HI-FCS supplemented with GM-CSF at 100 ng/mL (□ and ■) or a cytokine cocktail (50 ng/mL IL-6, 100 ng/mL SCF, and 10 ng/mL G-CSF;  or ▨). At the indicated days, cells were counted, assessed for viability, and plated in agar for a further 10 to 14 days in cocktail containing SCF, Epo, and IL-6. Error bars represent SEM from 3 to 5 replicate plates.
or ▨). At the indicated days, cells were counted, assessed for viability, and plated in agar for a further 10 to 14 days in cocktail containing SCF, Epo, and IL-6. Error bars represent SEM from 3 to 5 replicate plates.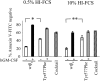


Similar articles
-
Tyrosine phosphorylation of Shc is not required for proliferation or viability signaling by granulocyte-macrophage colony-stimulating factor in hematopoietic cell lines.J Immunol. 1996 Jul 15;157(2):534-40. J Immunol. 1996. PMID: 8752899
-
Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2, SHIP, and phosphatidylinositol 3-kinase.Blood. 1999 Apr 15;93(8):2578-85. Blood. 1999. PMID: 10194437
-
Evidence for a physical association between the Shc-PTB domain and the beta c chain of the granulocyte-macrophage colony-stimulating factor receptor.J Biol Chem. 1996 May 24;271(21):12137-40. doi: 10.1074/jbc.271.21.12137. J Biol Chem. 1996. PMID: 8647804
-
Roles of JAK kinases in human GM-CSF receptor signal transduction.J Allergy Clin Immunol. 1996 Dec;98(6 Pt 2):S183-91. doi: 10.1016/s0091-6749(96)70065-9. J Allergy Clin Immunol. 1996. PMID: 8977526 Review.
-
CSF-1 signal transduction.J Leukoc Biol. 1997 Aug;62(2):145-55. doi: 10.1002/jlb.62.2.145. J Leukoc Biol. 1997. PMID: 9261328 Review.
Cited by
-
The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease.Blood. 2009 Aug 13;114(7):1289-98. doi: 10.1182/blood-2008-12-164004. Epub 2009 May 12. Blood. 2009. PMID: 19436055 Free PMC article. Review.
-
Distinct Assemblies of Heterodimeric Cytokine Receptors Govern Stemness Programs in Leukemia.Cancer Discov. 2023 Aug 4;13(8):1922-1947. doi: 10.1158/2159-8290.CD-22-1396. Cancer Discov. 2023. PMID: 37191437 Free PMC article.
-
The proximal signaling network of the BCR-ABL1 oncogene shows a modular organization.Oncogene. 2010 Nov 4;29(44):5895-910. doi: 10.1038/onc.2010.331. Epub 2010 Aug 9. Oncogene. 2010. PMID: 20697350 Free PMC article.
-
Loss of GM-CSF signalling in non-haematopoietic cells increases NSAID ileal injury.Gut. 2010 Aug;59(8):1066-78. doi: 10.1136/gut.2009.203893. Epub 2010 Jun 28. Gut. 2010. PMID: 20584783 Free PMC article.
-
Suppressor of Cytokine Signaling (SOCS) 5 utilises distinct domains for regulation of JAK1 and interaction with the adaptor protein Shc-1.PLoS One. 2013 Aug 21;8(8):e70536. doi: 10.1371/journal.pone.0070536. eCollection 2013. PLoS One. 2013. PMID: 23990909 Free PMC article.
References
-
- Nicola NA. Cytokine pleiotropy and redundancy: a view from the receptor. Stem Cells. 1994;12:3–12. - PubMed
-
- Guthridge MA, Stomski FC, Thomas D, et al. Mechanism of activation of the GM-CSF, IL-3 and IL-5 family of receptors. Stem Cells. 1998;16:301–313. - PubMed
-
- Reddy EP, Korapati A, Chaturvedi P, Rane S. IL-3 signaling and the role of Src kinases, JAKs and STATs: a covert liaison unveiled. Oncogene. 2000;19:2532–2547. - PubMed
-
- Bone H, Dechert U, Jirik F, Schrader JW, Welham MJ. SHP1 and SHP2 protein-tyrosine phosphatases associate with betac after interleukin-3-induced receptor tyrosine phosphorylation: identification of potential binding sites and substrates. J Biol Chem. 1997;272:14470–14476. - PubMed
-
- Pratt JC, Weiss M, Sieff CA, et al. Evidence for a physical association between the Shc-PTB domain and the beta c chain of the granulocyte-macrophage colony-stimulating factor receptor. J Biol Chem. 1996;271:12137–12140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

