Role of epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin
- PMID: 17638855
- PMCID: PMC2200921
- DOI: 10.1182/blood-2007-02-076091
Role of epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin
Abstract
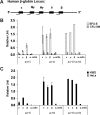
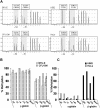
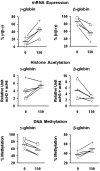
Butyrate is a prototype of histone deacetylase inhibitors that is believed to reactivate silent genes by inducing epigenetic modifications. Although butyrate was shown to induce fetal hemoglobin (HbF) production in patients with hemoglobin disorders, the mechanism of this induction has not been fully elucidated. Our studies of the epigenetic configuration of the beta-globin cluster suggest that DNA methylation and histone H3 acetylation are important for the regulation of developmental stage-specific expression of the beta-like globin genes, whereas acetylation of both histones H3 and H4 seem to be important for the regulation of tissue-specific expression. These studies suggest that DNA methylation may be important for the silencing of the beta-like globin genes in nonerythroid hematopoietic cells but may not be necessary for their silencing in nonhematopoietic cells. Furthermore, our studies demonstrate that butyrate exposure results in a true reversal of the normal developmental switch from gamma- to beta-globin expression. This is associated with increased histone acetylation and decreased DNA methylation of the gamma-globin genes, with opposite changes in the beta-globin gene. These studies provide strong support for the role of epigenetic modifications in the normal developmental and tissue-specific regulation of globin gene expression and in the butyrate-mediated pharmacologic induction of HbF production.
Figures


Similar articles
-
Epigenetic analysis of the human alpha- and beta-globin gene clusters.Blood Cells Mol Dis. 2008 Mar-Apr;40(2):166-73. doi: 10.1016/j.bcmd.2007.08.001. Epub 2007 Oct 29. Blood Cells Mol Dis. 2008. PMID: 18029204 Free PMC article.
-
Plastrum testudinis induces γ-globin gene expression through epigenetic histone modifications within the γ-globin gene promoter via activation of the p38 MAPK signaling pathway.Int J Mol Med. 2013 Jun;31(6):1418-28. doi: 10.3892/ijmm.2013.1338. Epub 2013 Apr 8. Int J Mol Med. 2013. PMID: 23588991
-
[Effects of sodium butyrate on acetylation of histone in gamma-globin gene promoter regions in K562 cells: a study using chromatin immunoprecipitation].Nan Fang Yi Ke Da Xue Xue Bao. 2010 Jun;30(6):1222-5. Nan Fang Yi Ke Da Xue Xue Bao. 2010. PMID: 20584642 Chinese.
-
Epigenetic regulation of fetal globin gene expression in adult erythroid cells.Transl Res. 2015 Jan;165(1):115-25. doi: 10.1016/j.trsl.2014.05.002. Epub 2014 May 11. Transl Res. 2015. PMID: 24880147 Free PMC article. Review.
-
Butyrate-induced reactivation of the fetal globin genes: a molecular treatment for the beta-hemoglobinopathies.Experientia. 1993 Feb 15;49(2):133-7. doi: 10.1007/BF01989417. Experientia. 1993. PMID: 7680003 Review.
Cited by
-
In Vitro and In Vivo Studies for the Investigation of γ-Globin Gene Induction by Adhatoda vasica: A Pre-Clinical Study of HbF Inducers for β-Thalassemia.Front Pharmacol. 2022 Mar 29;13:797853. doi: 10.3389/fphar.2022.797853. eCollection 2022. Front Pharmacol. 2022. PMID: 35422700 Free PMC article.
-
Fetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathies.Mol Cell Biol. 2014 Oct 1;34(19):3560-9. doi: 10.1128/MCB.00714-14. Epub 2014 Jul 14. Mol Cell Biol. 2014. PMID: 25022757 Free PMC article. Review.
-
Epigenetic activities in erythroid cell gene regulation.Semin Hematol. 2021 Jan;58(1):4-9. doi: 10.1053/j.seminhematol.2020.11.007. Epub 2020 Dec 15. Semin Hematol. 2021. PMID: 33509442 Free PMC article.
-
Transcriptional activation of the gamma-globin gene in baboons treated with decitabine and in cultured erythroid progenitor cells involves different mechanisms.Exp Hematol. 2009 Oct;37(10):1131-42. doi: 10.1016/j.exphem.2009.06.007. Epub 2009 Jul 2. Exp Hematol. 2009. PMID: 19576949 Free PMC article.
-
Differences in response to fetal hemoglobin induction therapy in beta-thalassemia and sickle cell disease.Blood Cells Mol Dis. 2009 Jul-Aug;43(1):58-62. doi: 10.1016/j.bcmd.2009.02.006. Epub 2009 Apr 5. Blood Cells Mol Dis. 2009. PMID: 19346141 Free PMC article.
References
-
- Harju S, McQueen KJ, Peterson KR. Chromatin structure and control of beta-like globin gene switching. Exp Biol Med (Maywood) 2002;227:683–700. - PubMed
-
- Bulger M, Sawado T, Schubeler D, Groudine M. ChIPs of the beta-globin locus: unraveling gene regulation within an active domain. Curr Opin Genet Dev. 2002;12:170–177. - PubMed
-
- Forsberg EC, Bresnick EH. Histone acetylation beyond promoters: long-range acetylation patterns in the chromatin world. Bioessays. 2001;23:820–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

