Small interfering RNA directed reversal of urokinase plasminogen activator demethylation inhibits prostate tumor growth and metastasis
- PMID: 17638874
- PMCID: PMC2390768
- DOI: 10.1158/0008-5472.CAN-07-0751
Small interfering RNA directed reversal of urokinase plasminogen activator demethylation inhibits prostate tumor growth and metastasis
Retraction in
-
Retraction: Small Interfering RNA-Directed Reversal of Urokinase Plasminogen Activator Demethylation Inhibits Prostate Tumor Growth and Metastasis.Cancer Res. 2018 Jun 15;78(12):3398. doi: 10.1158/0008-5472.CAN-18-1183. Cancer Res. 2018. PMID: 29907683 Free PMC article. No abstract available.
Abstract
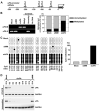
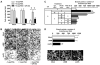
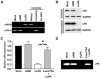
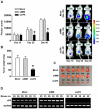
Recent studies have shown that small interfering RNA (siRNA) silences genes at the transcriptional level in human cells. However, the therapeutic potential of siRNA-mediated transcriptional gene silencing remains unclear. Here, we show that siRNA targeted to the urokinase plasminogen activator (uPA) promoter induced epigenetic transcriptional silencing in human prostate cancer cells. This silencing resulted in a dramatic reduction of tumor cell invasion and angiogenesis in vitro. Furthermore, the results from a bioluminescence tumor/metastasis model showed that the silencing of uPA significantly inhibits prostate tumor growth and the incidence of lung metastasis. Our findings represent a potentially powerful new approach to not only epigenetic silencing of metastasis or growth-promoting genes as a cancer therapy, but also as a means to shed light on how aberrant de novo methylation during cancer progression might be targeted to specific sequences.
Figures
Similar articles
-
RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo.J Biol Chem. 2005 Oct 28;280(43):36529-40. doi: 10.1074/jbc.M503111200. Epub 2005 Aug 26. J Biol Chem. 2005. Retraction in: J Biol Chem. 2020 Sep 11;295(37):13136. doi: 10.1074/jbc.RX120.015588. PMID: 16127174 Free PMC article. Retracted.
-
Demethylation-linked activation of urokinase plasminogen activator is involved in progression of prostate cancer.Cancer Res. 2007 Feb 1;67(3):930-9. doi: 10.1158/0008-5472.CAN-06-2892. Cancer Res. 2007. Retraction in: Cancer Res. 2018 Jun 15;78(12):3397. doi: 10.1158/0008-5472.CAN-18-1182. PMID: 17283123 Free PMC article. Retracted.
-
Prostate cancer cell-derived urokinase-type plasminogen activator contributes to intraosseous tumor growth and bone turnover.Neoplasia. 2008 May;10(5):439-49. doi: 10.1593/neo.08106. Neoplasia. 2008. PMID: 18472961 Free PMC article.
-
The urokinase-type plasminogen activator system in prostate cancer metastasis.Cancer Metastasis Rev. 2001;20(3-4):287-96. doi: 10.1023/a:1015539612576. Cancer Metastasis Rev. 2001. PMID: 12085967 Review.
-
Transforming Growth Factor-Beta and Urokinase Type Plasminogen Interplay in Cancer.Curr Protein Pept Sci. 2018;19(12):1155-1163. doi: 10.2174/1389203718666171030103801. Curr Protein Pept Sci. 2018. PMID: 29086689 Review.
Cited by
-
Multimodality imaging of RNA interference.Curr Med Chem. 2013;20(29):3664-75. doi: 10.2174/0929867311320290012. Curr Med Chem. 2013. PMID: 23745567 Free PMC article. Review.
-
Non-coding RNAs, epigenetic memory and the passage of information to progeny.RNA Biol. 2009 Jul-Aug;6(3):242-7. doi: 10.4161/rna.6.3.8353. Epub 2009 Jul 5. RNA Biol. 2009. PMID: 19305164 Free PMC article.
-
Transcriptional gene silencing in mammalian cells by miRNA mimics that target gene promoters.Nucleic Acids Res. 2011 Jul;39(13):5682-91. doi: 10.1093/nar/gkr155. Epub 2011 Mar 22. Nucleic Acids Res. 2011. PMID: 21427083 Free PMC article.
-
Retraction: Small Interfering RNA-Directed Reversal of Urokinase Plasminogen Activator Demethylation Inhibits Prostate Tumor Growth and Metastasis.Cancer Res. 2018 Jun 15;78(12):3398. doi: 10.1158/0008-5472.CAN-18-1183. Cancer Res. 2018. PMID: 29907683 Free PMC article. No abstract available.
-
Promoter-associated RNAs and promoter-targeted RNAs.Cell Mol Life Sci. 2012 Sep;69(17):2833-42. doi: 10.1007/s00018-012-0953-1. Epub 2012 Mar 14. Cell Mol Life Sci. 2012. PMID: 22415323 Free PMC article. Review.
References
-
- Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–8. - PubMed
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. - PubMed
-
- Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Genet. 2001;2:110–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

