Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis
- PMID: 17639077
- PMCID: PMC1920165
- DOI: 10.1101/gad.1549407
Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis
Abstract
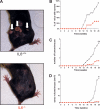
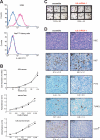
Ras is mutated to remain in the active oncogenic state in many cancers. As Ras has proven difficult to target therapeutically, we searched for secreted, druggable proteins induced by Ras that are required for tumorigenesis. We found that Ras induces the secretion of cytokine IL6 in different cell types, and that knockdown of IL6, genetic ablation of the IL6 gene, or treatment with a neutralizing IL6 antibody retard Ras-driven tumorigenesis. IL6 appears to act in a paracrine fashion to promote angiogenesis and tumor growth. Inhibiting IL6 may therefore have therapeutic utility for treatment of cancers characterized by oncogenic Ras mutations.
Figures


Comment in
-
Targeting oncogenic Ras.Genes Dev. 2007 Aug 15;21(16):1989-92. doi: 10.1101/gad.1587907. Genes Dev. 2007. PMID: 17699748 Review. No abstract available.
References
-
- Aarden L.A., De Groot E.R., Schaap O.L., Lansdorp P.M., De Groot E.R., Schaap O.L., Lansdorp P.M., Schaap O.L., Lansdorp P.M., Lansdorp P.M. Production of hybridoma growth factor by human monocytes. Eur. J. Immunol. 1987;17:1411–1416. - PubMed
-
- Adams G.P., Weiner L.M., Weiner L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005;23:1147–1157. - PubMed
-
- Barber M.D., Fearon K.C., Ross J.A., Fearon K.C., Ross J.A., Ross J.A. Relationship of serum levels of interleukin-6, soluble interleukin-6 receptor and tumour necrosis factor receptors to the acute-phase protein response in advanced pancreatic cancer. Clin. Sci. 1999;96:83–87. - PubMed
-
- Cohen T., Nahari D., Cerem L.W., Neufeld G., Levi B.Z., Nahari D., Cerem L.W., Neufeld G., Levi B.Z., Cerem L.W., Neufeld G., Levi B.Z., Neufeld G., Levi B.Z., Levi B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996;271:736–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
