TGF-beta signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis
- PMID: 17639085
- PMCID: PMC1920175
- DOI: 10.1101/gad.1541807
TGF-beta signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis
Abstract
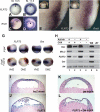
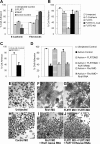
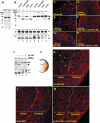
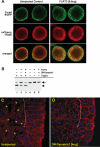
The molecular mechanisms governing the cell behaviors underlying morphogenesis remain a major focus of research in both developmental biology and cancer biology. TGF-beta ligands control cell fate specification via Smad-mediated signaling. However, their ability to guide cellular morphogenesis in a variety of biological contexts is poorly understood. We report on the discovery of a novel TGF-beta signaling-mediated cellular morphogenesis occurring during vertebrate gastrulation. Activin/nodal members of the TGF-beta superfamily induce the expression of two genes regulating cell adhesion during gastrulation: Fibronectin Leucine-rich Repeat Transmembrane 3 (FLRT3), a type I transmembrane protein containing extracellular leucine-rich repeats, and the small GTPase Rnd1. FLRT3 and Rnd1 interact physically and modulate cell adhesion during embryogenesis by controlling cell surface levels of cadherin through a dynamin-dependent endocytosis pathway. Our model suggests that cell adhesion can be dynamically regulated by sequestering cadherin through internalization, and subsequent redeploying internalized cadherin to the cell surface as needed. As numerous studies have linked aberrant expression of small GTPases, adhesion molecules such as cadherins, and TGF-beta signaling to oncogenesis and metastasis, it is tempting to speculate that this FLRT3/Rnd1/cadherin pathway might also control cell behavior and morphogenesis in adult tissue homeostasis.
Figures



References
-
- Asashima M., Nakano H., Shimada K., Kinoshita K., Ishii K., Shibai H., Ueno N., Nakano H., Shimada K., Kinoshita K., Ishii K., Shibai H., Ueno N., Shimada K., Kinoshita K., Ishii K., Shibai H., Ueno N., Kinoshita K., Ishii K., Shibai H., Ueno N., Ishii K., Shibai H., Ueno N., Shibai H., Ueno N., Ueno N. Mesodermal induction in early amphibian embryos by activin A (erythroid differentiation factor) Rouxs Arch. Dev. Biol. 1990;198:330–335. - PubMed
-
- Blitz I.L., Shimmi O., Wunnenberg-Stapleton K., O’Connor M.B., Cho K.W., Shimmi O., Wunnenberg-Stapleton K., O’Connor M.B., Cho K.W., Wunnenberg-Stapleton K., O’Connor M.B., Cho K.W., O’Connor M.B., Cho K.W., Cho K.W. Is chordin a long-range- or short-range-acting factor? Roles for BMP1-related metalloproteases in chordin and BMP4 autofeedback loop regulation. Dev. Biol. 2000;223:120–138. - PubMed
-
- Bottcher R.T., Pollet N., Delius H., Niehrs C., Pollet N., Delius H., Niehrs C., Delius H., Niehrs C., Niehrs C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat. Cell Biol. 2004;6:38–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
