Efficacy of nonviral gene transfer in the canine brain
- PMID: 17639883
- PMCID: PMC2384235
- DOI: 10.3171/JNS-07/07/0136
Efficacy of nonviral gene transfer in the canine brain
Abstract
Object: The purpose of this study was to evaluate the gene transfer capability and tolerability of plasmid DNA/polyethylenimine (PEI) complexes in comparison with adenovirus and naked plasmid DNA in the canine brain.
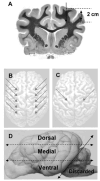
Methods: Plasmid or adenoviral vectors encoding firefly luciferase were injected directly into the cerebral parenchyma of five adult dogs at varying doses and volumes. Serial physical and neurological examinations, as well as blood and cerebrospinal fluid (CSF) analyses, were conducted before and after the surgery for 3 days. Three days after gene delivery, a luciferase activity assay and immunofluorescence analysis were used to test the brain tissue for gene expression.
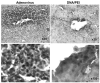
Results: Injection into the brain parenchyma resulted in gene transfer throughout the cerebrum with every vector tested. Luciferase expression was highest when adenovirus vectors were used. Injection of plasmid DNA/PEI complexes and naked DNA resulted in similar levels of luciferase expression, which were on average 0.5 to 1.5% of the expression achieved with adenovirus vectors. Immunofluorescent microscopy analysis revealed that plasmid DNA/PEI complexes transduced mainly neurons, whereas adenovirus transduced mainly astrocytes. No significant acute side effects or neurological complications were observed in any of the dogs. Mononuclear cell counts significantly increased in the CSF after adenovirus injection and modestly increased after injection of plasmid DNA/PEI complexes, suggesting that a mild, acute inflammatory response occurred in the central nervous system (CNS).
Conclusions: Compared with rodent models that are limited by very small brains, the dog is an excellent preclinical model in which to assess the distribution and safety of emerging gene transfer technologies. In this study, short-term gene transfer was evaluated as a prelude to long-term expression and safety studies. The authors conclude that the viral and nonviral vectors tested were well tolerated and effective at mediating gene transfer throughout a large portion of the canine brain. The nonviral plasmid vectors were less effective than adenovirus, yet they still achieved appreciable gene expression levels. Due to reduced gene transfer efficiency relative to viral vectors, nonviral vectors may be most useful when the expressed protein is secreted or exerts a bystander effect. Nonviral vectors offer an alternative means to genetically modify cells within the CNS of large mammals.
Figures



Similar articles
-
Tumor-directed gene therapy in mice using a composite nonviral gene delivery system consisting of the piggyBac transposon and polyethylenimine.BMC Cancer. 2009 Apr 27;9:126. doi: 10.1186/1471-2407-9-126. BMC Cancer. 2009. PMID: 19397814 Free PMC article.
-
CNS gene transfer mediated by a novel controlled release system based on DNA complexes of degradable polycation PPE-EA: a comparison with polyethylenimine/DNA complexes.Gene Ther. 2004 Jan;11(1):109-14. doi: 10.1038/sj.gt.3302135. Gene Ther. 2004. PMID: 14681704
-
Improvement of nonviral gene therapy by Epstein-Barr virus (EBV)-based plasmid vectors.Curr Gene Ther. 2002 Sep;2(3):379-92. doi: 10.2174/1566523023347814. Curr Gene Ther. 2002. PMID: 12189722 Review.
-
Evaluation of helper-dependent canine adenovirus vectors in a 3D human CNS model.Gene Ther. 2016 Jan;23(1):86-94. doi: 10.1038/gt.2015.75. Epub 2015 Aug 20. Gene Ther. 2016. PMID: 26181626 Free PMC article.
-
The conundrum between immunological memory to adenovirus and their use as vectors in clinical gene therapy.Mol Biotechnol. 2006 Oct;34(2):247-56. doi: 10.1385/MB:34:2:247. Mol Biotechnol. 2006. PMID: 17172670 Review.
Cited by
-
Nanoparticles for retinal gene therapy.Prog Retin Eye Res. 2010 Sep;29(5):376-97. doi: 10.1016/j.preteyeres.2010.04.004. Epub 2010 May 7. Prog Retin Eye Res. 2010. PMID: 20452457 Free PMC article. Review.
-
Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety.Curr Gene Ther. 2009 Oct;9(5):409-21. doi: 10.2174/156652309789753301. Curr Gene Ther. 2009. PMID: 19860655 Free PMC article. Review.
-
Advances in diagnostic and treatment modalities for intracranial tumors.J Vet Intern Med. 2014 Jul-Aug;28(4):1165-85. doi: 10.1111/jvim.12370. Epub 2014 May 9. J Vet Intern Med. 2014. PMID: 24814688 Free PMC article. Review.
-
Victory and defeat in the induction of a therapeutic response through vaccine therapy for human and canine brain tumors: a review of the state of the art.Crit Rev Immunol. 2014;34(5):399-432. doi: 10.1615/critrevimmunol.2014011577. Crit Rev Immunol. 2014. PMID: 25404047 Free PMC article. Review.
-
Long-term transgene expression in the central nervous system using DNA nanoparticles.Mol Ther. 2009 Apr;17(4):641-50. doi: 10.1038/mt.2009.2. Epub 2009 Feb 17. Mol Ther. 2009. PMID: 19223866 Free PMC article.
References
-
- Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996;7:1947–1954. - PubMed
-
- Ambar BB, Frei K, Malipiero U, Morelli AE, Castro MG, Lowenstein PR, et al. Treatment of experimental glioma by administration of adenoviral vectors expressing Fas ligand. Hum Gene Ther. 1999;10:1641–1648. - PubMed
-
- Banerjee P, Weissleder R, Bogdanov A., Jr Linear polyethyleneimine grafted to a hyperbranched poly(ethylene glycol)-like core: a copolymer for gene delivery. Bioconjug Chem. 2006;17:125–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

