Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer
- PMID: 17640394
- PMCID: PMC2206709
- DOI: 10.1186/bcr1678
Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer
Abstract
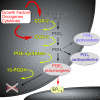
Many human cancers exhibit elevated prostaglandin (PG) levels due to upregulation of cyclooxygenase-2 (COX-2), a key enzyme in eicosanoid biosynthesis. COX-2 over-expression has been observed in about 40% of cases of invasive breast carcinoma and at a higher frequency in preinvasive ductal carcinoma in situ tumors, Extensive pharmacologic and genetic evidence implicates COX enzymes in neoplasia. Epidemiologic analyses demonstrate a protective effect of COX-inhibiting nonsteroidal anti-inflammatory drugs with respect to human cancer. Complementary experimental studies have established that both conventional nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors suppress mammary tumor formation in rodent breast cancer models. Furthermore, knocking out Cox-2 reduces mammary tumorigenesis and angiogenesis, and, conversely, transgenic COX-2 over-expression induces tumor formation. The utility of COX/PG signaling as a target for chemoprevention has been established by randomized controlled clinical trials. However, these studies also identified increased cardiovascular risk associated with use of selective COX-2 inhibitors. Thus, current efforts are directed toward identifying safer approaches to antagonizing COX/PG signaling for cancer prevention and treatment, with a particular focus on PGE2 regulation and signaling, because PGE2 is a key pro-tumorigenic prostanoid.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

