Mechanisms for persistent microphthalmia following ethanol exposure during retinal neurogenesis in zebrafish embryos
- PMID: 17640445
- PMCID: PMC2583023
- DOI: 10.1017/S0952523807070423
Mechanisms for persistent microphthalmia following ethanol exposure during retinal neurogenesis in zebrafish embryos
Abstract
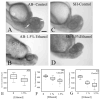
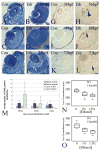
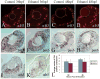
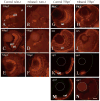
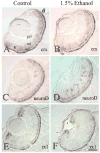
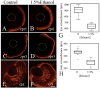
The exposure of the developing human embryo to ethanol results in a spectrum of disorders involving multiple organ systems, including the visual system. One common phenotype seen in humans exposed to ethanol in utero is microphthalmia. The objective of this study was to describe the effects of ethanol during retinal neurogenesis in a model organism, the zebrafish, and to pursue the potential mechanisms by which ethanol causes microphthalmia. Zebrafish embryos were exposed to 1% or 1.5% ethanol from 24 to 48 h after fertilization, a period during which the retinal neuroepithelium undergoes rapid proliferation and differentiation to form a laminated structure composed of different retinal cell types. Ethanol exposure resulted in significantly reduced eye size immediately following the treatment, and this microphthalmia persisted through larval development. This reduced eye size could not entirely be accounted for by the accompanying general delay in embryonic development. Retinal cell death was only slightly higher in ethanol-exposed embryos, although cell death in the lens was extensive in some of these embryos, and lenses were significantly reduced in size as compared to those of control embryos. The initiation of retinal neurogenesis was not affected, but the subsequent waves of cell differentiation were markedly reduced. Even cells that were likely generated after ethanol exposure--rod and cone photoreceptors and Müller glia--were delayed in their expression of cell-specific markers by at least 24 h. We conclude that ethanol exposure over the time of retinal neurogenesis resulted in persistent microphthalmia due to a combination of an overall developmental delay, lens abnormalities, and reduced retinal cell differentiation.
Figures

References
-
- Arenzana FJ, Carvan MJ, 3rd, Aijon J, Sanchez-Gonzalez R, Arevalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicology and Teratology. 2006;28:342–348. - PubMed
-
- Barthel LK, Raymond PA. Subcellular localization of alpha-tubulin and opsin mRNA in the goldfish retina using digoxigenin-labeled cRNA probes detected by alkaline phosphatase and HRP histochemistry. Journal of Neuroscience Methods. 1993;50:145–152. - PubMed
-
- Biehlmaier O, Neuhauss SC, Kohler K. Onset and time course of apoptosis in the developing zebrafish retina. Cell Tissue Research. 2001;306:199–207. - PubMed
-
- Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: A novel model of fetal alcohol syndrome. Neurotoxicology and Teratology. 2004;26:737–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

