AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling
- PMID: 17640527
- PMCID: PMC2698451
- DOI: 10.1016/j.neuron.2007.06.032
AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling
Abstract
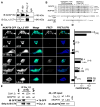
Neuronal L-type calcium channels contribute to dendritic excitability and activity-dependent changes in gene expression that influence synaptic strength. Phosphorylation-mediated enhancement of L-type channels containing the CaV1.2 pore-forming subunit is promoted by A-kinase anchoring proteins (AKAPs) that target cAMP-dependent protein kinase (PKA) to the channel. Although PKA increases L-type channel activity in dendrites and dendritic spines, the mechanism of enhancement in neurons remains poorly understood. Here, we show that CaV1.2 interacts directly with AKAP79/150, which binds both PKA and the Ca2+/calmodulin-activated phosphatase calcineurin (CaN). Cotargeting of PKA and CaN by AKAP79/150 confers bidirectional regulation of L-type current amplitude in transfected HEK293 cells and hippocampal neurons. However, anchored CaN dominantly suppresses PKA enhancement of the channel. Additionally, activation of the transcription factor NFATc4 via local Ca2+ influx through L-type channels requires AKAP79/150, suggesting that this signaling complex promotes neuronal L channel signaling to the nucleus through NFATc4.
Figures
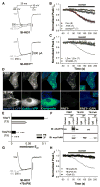
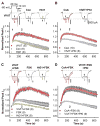
 (Con), Current down-regulation in response to St-Ht31 in cells transfected with wild type CaV1.2 and AKAP79.
(Con), Current down-regulation in response to St-Ht31 in cells transfected with wild type CaV1.2 and AKAP79. (-FSK), Effect of St-Ht31 in the absence of FSK.
(-FSK), Effect of St-Ht31 in the absence of FSK.  , (St-Ht31pro) Response to application of a proline-substituted form of St-Ht31. (C) St-Ht31 application to cells coexpressing either LZm forms CaV1.2 and AKAP79 (LZm/LZm,
, (St-Ht31pro) Response to application of a proline-substituted form of St-Ht31. (C) St-Ht31 application to cells coexpressing either LZm forms CaV1.2 and AKAP79 (LZm/LZm,  ), or the CaV1.2 phosphorylation site mutant S1928A and wild-type AKAP79 (S1928A,
), or the CaV1.2 phosphorylation site mutant S1928A and wild-type AKAP79 (S1928A,  ). (D) Representative micrographs of COS7 cells demonstrating subcellular localization and 3-filter sensitized FRETC (presented as in Figure 1C) between CFP-labeled AKAP79 (79WT, top) or AKAP79 lacking the core PXIXIT-like motif of the CaN binding site (79ΔPIX, bottom) and CaN A-YFP. (E) Effective membrane FRET efficiencies (Eeff) for AKAP79WT-CFP or AKAP79ΔPIX-CFP and CaN A-YFP as measured by acceptor photobleaching (PB) and 3-filter (3F) methods (n values indicated in parentheses; *** P = 1.38 × 10−6 and ### P = 2.16 × 10−7). (F) Top, immunoprecipitations (IP) of myc-tagged CaNA (α-myc) from lysates of HEK293 cells transfected with myc-CaNA and either 79WT, 79ΔPIX or AKAP79ΔCaN, probed with anti-AKAP79. Bottom, the same blot was stripped of anti-AKAP79 and re-probed with anti-myc (n = 3 for both panels). (G) Representative current records from HEK293 cells expressing 79ΔPIX-YFP and wild type CaV1.2 exposed to St-Ht31 beginning at 0 s. (H) Average peak current time course following St-Ht31 application to cells expressing wild type CaV1.2 and 79ΔCaN-YFP(
). (D) Representative micrographs of COS7 cells demonstrating subcellular localization and 3-filter sensitized FRETC (presented as in Figure 1C) between CFP-labeled AKAP79 (79WT, top) or AKAP79 lacking the core PXIXIT-like motif of the CaN binding site (79ΔPIX, bottom) and CaN A-YFP. (E) Effective membrane FRET efficiencies (Eeff) for AKAP79WT-CFP or AKAP79ΔPIX-CFP and CaN A-YFP as measured by acceptor photobleaching (PB) and 3-filter (3F) methods (n values indicated in parentheses; *** P = 1.38 × 10−6 and ### P = 2.16 × 10−7). (F) Top, immunoprecipitations (IP) of myc-tagged CaNA (α-myc) from lysates of HEK293 cells transfected with myc-CaNA and either 79WT, 79ΔPIX or AKAP79ΔCaN, probed with anti-AKAP79. Bottom, the same blot was stripped of anti-AKAP79 and re-probed with anti-myc (n = 3 for both panels). (G) Representative current records from HEK293 cells expressing 79ΔPIX-YFP and wild type CaV1.2 exposed to St-Ht31 beginning at 0 s. (H) Average peak current time course following St-Ht31 application to cells expressing wild type CaV1.2 and 79ΔCaN-YFP( ) or 79ΔPIX-YFP (
) or 79ΔPIX-YFP ( ), or wild type CaV1.2 and AKAP79-YFP in the presence of the CaN inhibitor cyclosporin A (CsA, ◆). In C and H, small dots reproduce the time course of St-Ht31 action in the control condition shown in B as
), or wild type CaV1.2 and AKAP79-YFP in the presence of the CaN inhibitor cyclosporin A (CsA, ◆). In C and H, small dots reproduce the time course of St-Ht31 action in the control condition shown in B as  .
.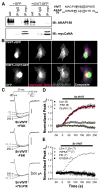
 (Con), Current up-regulation in response to St-VIVIT in cells transfected with wild type CaV1.2 and AKAP79.
(Con), Current up-regulation in response to St-VIVIT in cells transfected with wild type CaV1.2 and AKAP79.  (-FSK), Effect of St-VIVIT in the absence of FSK.
(-FSK), Effect of St-VIVIT in the absence of FSK.  (79ΔPIX) Effect of St-VIVIT in cells expressing 79ΔPIX-YFP. (E) St-VIVIT application to cells expressing LZm forms of both CaV1.2 and AKAP79 (LZm/LZm, ◇), wild type CaV1.2 and a PKA binding-deficient AKAP79 containing A396P and I404P mutations (79pro2,
(79ΔPIX) Effect of St-VIVIT in cells expressing 79ΔPIX-YFP. (E) St-VIVIT application to cells expressing LZm forms of both CaV1.2 and AKAP79 (LZm/LZm, ◇), wild type CaV1.2 and a PKA binding-deficient AKAP79 containing A396P and I404P mutations (79pro2,  ), the CaV1.2 phosphorylation site mutant S1928A and wild type AKAP79 (S1928A,
), the CaV1.2 phosphorylation site mutant S1928A and wild type AKAP79 (S1928A,  ), or to cells expressing wild type CaV1.2 and AKAP79 preincubated with the PKA inhibitor PKI (PKI,
), or to cells expressing wild type CaV1.2 and AKAP79 preincubated with the PKA inhibitor PKI (PKI,  ). Small dots reproduce the control condition shown in D as
). Small dots reproduce the control condition shown in D as  .
.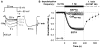
 ) or in the presence of either VIVIT (
) or in the presence of either VIVIT ( ) or Ht31 (
) or Ht31 ( ). (B) The effect of CsA dialysis (
). (B) The effect of CsA dialysis ( ) and co-application of VIVIT and PKI (
) and co-application of VIVIT and PKI ( ). (C and D) as in A and B, but with FSK added to the intracellular recording solution.
). (C and D) as in A and B, but with FSK added to the intracellular recording solution.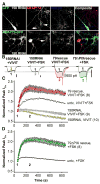
 ) or co-application of VIVIT and FSK (150RNAi, VIVIT + FSK,
) or co-application of VIVIT and FSK (150RNAi, VIVIT + FSK,  ), and for cells rescued from AKAP150 knockdown by AKAP79-GFP co-transfection (79 rescue, VIVIT + FSK,
), and for cells rescued from AKAP150 knockdown by AKAP79-GFP co-transfection (79 rescue, VIVIT + FSK,  ). Small dots reproduce the effect of VIVIT + FSK observed in untransfected neurons (
). Small dots reproduce the effect of VIVIT + FSK observed in untransfected neurons ( from Fig. 4C). (D) The effect of FSK alone on neurons after AKAP150 knockdown and replacement with AKAP79ΔPIX-YFP (79ΔPIX rescue, +FSK,
from Fig. 4C). (D) The effect of FSK alone on neurons after AKAP150 knockdown and replacement with AKAP79ΔPIX-YFP (79ΔPIX rescue, +FSK,  ). Small dots reproduce the effect of FSK in untransfected neurons (
). Small dots reproduce the effect of FSK in untransfected neurons ( from Figure 4C).
from Figure 4C).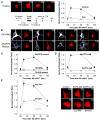

References
-
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan G, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. - PubMed
-
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Bauman AL, Goehring AS, Scott JD. Orchestration of synaptic plasticity through AKAP signaling complexes. Neuropharmacology. 2004;46:299–310. - PubMed
-
- Bean BP, Nowycky MC, Tsien RW. β-adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

