Functional characterization of novel telomerase RNA (TERC) mutations in patients with diverse clinical and pathological presentations
- PMID: 17640862
- PMCID: PMC2892775
- DOI: 10.3324/haematol.11407
Functional characterization of novel telomerase RNA (TERC) mutations in patients with diverse clinical and pathological presentations
Abstract
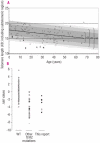
Background and objectives: Functional characterization of heterozygous TERC (telomerase RNA component) and TERT (telomerase reverse transcriptase) mutations found in autosomal dominant dyskeratosis congenita (DC) and aplastic anemia (AA) shows that telomerase function is defective and that this is associated with short telomeres. This leads to reduced cell longevity with maximal impact on tissues with high proliferate potential. The aim of this study was to establish the role of TERC in the pathophysiology of uncharacterized patients with AA with some features of DC.
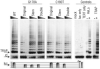
Design and methods: The TERC gene was screened for mutations by denaturing high performance liquid chromatography. To determine the functional significance of TERC mutations telomerase activity was assessed in an in vitro (TRAP) assay and telomere length of patients' samples was determined using Southern blot analysis. RESULTS This study led to the identification of four novel TERC mutations (G178A, C180T, D52-86 and G2C) and a recurrent TERC mutation (D110-113GACT).
Interpretation and conclusions: Two of the de novo TERC mutations (G178A and C180T) found uniquely produce a clinical phenotype in the first generation, differing from previously published cases in which individuals in the first generation are usually asymptomatic. Curiously these mutations are located near the triple-helix domain of TERC. We also observed that the recurrent D110-113GACT can present with AA, myelodysplasia or leukemia. The D52-86 is associated with varied phenotypes including pulmonary disease (pulmonary fibrosis) as the first presentation. In summary, this study reports the functional characterization of several novel TERC mutations associated with varied hematologic and extra-hematologic presentations.
Figures




Comment in
-
Dysfunctional telomeres and dyskeratosis congenita.Haematologica. 2007 Aug;92(8):1009-12. doi: 10.3324/haematol.11221. Haematologica. 2007. PMID: 17650438 No abstract available.
References
-
- Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–8. - PubMed
-
- Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–5. - PubMed
-
- Chen JL, Greider CW. Telomerase RNA structure and function: implications for dyskeratosis congenita. Trends Biochem Sci. 2004;29:183–92. - PubMed
-
- Marrone A, Dokal I. Dyskeratosis congenita: a disorder of telomerase deficiency and its relationship to other diseases. Expert Rev Derm. 2006;1:463–79.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

