Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells
- PMID: 17640868
- PMCID: PMC2377359
- DOI: 10.1074/jbc.M704055200
Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells
Abstract
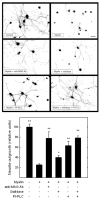
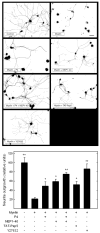
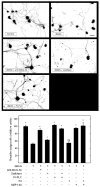
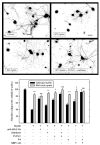
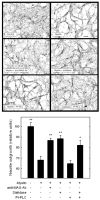
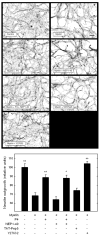
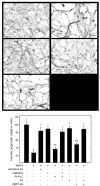
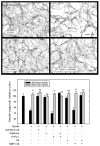
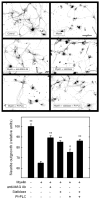
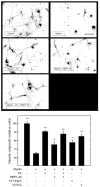
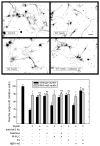
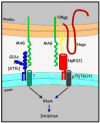
In the injured nervous system, myelin-associated glycoprotein (MAG) on residual myelin binds to receptors on axons, inhibits axon outgrowth, and limits functional recovery. Conflicting reports identify gangliosides (GD1a and GT1b) and glycosylphosphatidylinositol-anchored Nogo receptors (NgRs) as exclusive axonal receptors for MAG. We used enzymes and pharmacological agents to distinguish the relative roles of gangliosides and NgRs in MAG-mediated inhibition of neurite outgrowth from three nerve cell types, dorsal root ganglion neurons (DRGNs), cerebellar granule neurons (CGNs), and hippocampal neurons. Primary rat neurons were cultured on control substrata and substrata adsorbed with full-length native MAG extracted from purified myelin. The receptors responsible for MAG inhibition of neurite outgrowth varied with nerve cell type. In DRGNs, most of the MAG inhibition was via NgRs, evidenced by reversal of inhibition by phosphatidylinositol-specific phospholipase C (PI-PLC), which cleaves glycosylphosphatidylinositol anchors, or by NEP1-40, a peptide inhibitor of NgR. A smaller percentage of MAG inhibition of DRGN outgrowth was via gangliosides, evidenced by partial reversal by addition of sialidase to cleave GD1a and GT1b or by P4, an inhibitor of ganglioside biosynthesis. Combining either PI-PLC and sialidase or NEP1-40 and P4 was additive. In contrast to DRGNs, in CGNs MAG inhibition was exclusively via gangliosides, whereas inhibition of hippocampal neuron outgrowth was mostly reversed by sialidase or P4 and only modestly reversed by PI-PLC or NEP1-40 in a non-additive fashion. A soluble proteolytic fragment of native MAG, dMAG, also inhibited neurite outgrowth. In DRGNs, dMAG inhibition was exclusively NgR-dependent, whereas in CGNs it was exclusively ganglioside-dependent. An inhibitor of Rho kinase reversed MAG-mediated inhibition in all nerve cells, whereas a peptide inhibitor of the transducer p75(NTR) had cell-specific effects quantitatively similar to NgR blockers. Our data indicate that MAG inhibits axon outgrowth via two independent receptors, gangliosides and NgRs.
Figures
Similar articles
-
Myelin-associated glycoprotein modulates apoptosis of motoneurons during early postnatal development via NgR/p75(NTR) receptor-mediated activation of RhoA signaling pathways.Cell Death Dis. 2015 Sep 3;6(9):e1876. doi: 10.1038/cddis.2015.228. Cell Death Dis. 2015. PMID: 26335717 Free PMC article.
-
Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth.Nature. 2002 Jun 27;417(6892):941-4. doi: 10.1038/nature00867. Epub 2002 Jun 16. Nature. 2002. PMID: 12068310
-
Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition.J Biol Chem. 2001 Jun 8;276(23):20280-5. doi: 10.1074/jbc.M100345200. Epub 2001 Mar 6. J Biol Chem. 2001. PMID: 11279053
-
Myelin-associated glycoprotein and its axonal receptors.J Neurosci Res. 2009 Nov 15;87(15):3267-76. doi: 10.1002/jnr.21992. J Neurosci Res. 2009. PMID: 19156870 Free PMC article. Review.
-
Structural features of the Nogo receptor signaling complexes at the neuron/myelin interface.Neurosci Res. 2014 Oct;87:1-7. doi: 10.1016/j.neures.2014.06.003. Epub 2014 Jun 20. Neurosci Res. 2014. PMID: 24956133 Review.
Cited by
-
Ganglioside regulation of AMPA receptor trafficking.J Neurosci. 2014 Sep 24;34(39):13246-58. doi: 10.1523/JNEUROSCI.1149-14.2014. J Neurosci. 2014. PMID: 25253868 Free PMC article.
-
A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway.J Neurosci. 2010 Jan 13;30(2):739-48. doi: 10.1523/JNEUROSCI.5266-09.2010. J Neurosci. 2010. PMID: 20071539 Free PMC article.
-
Myelin-associated glycoprotein protects neurons from excitotoxicity.J Neurochem. 2011 Mar;116(5):900-8. doi: 10.1111/j.1471-4159.2010.07069.x. Epub 2011 Jan 7. J Neurochem. 2011. PMID: 21214567 Free PMC article.
-
Axonal regeneration after spinal cord injury in zebrafish and mammals: differences, similarities, translation.Neurosci Bull. 2013 Aug;29(4):402-10. doi: 10.1007/s12264-013-1361-8. Epub 2013 Jul 28. Neurosci Bull. 2013. PMID: 23893428 Free PMC article. Review.
-
Sialidase enhances recovery from spinal cord contusion injury.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11561-6. doi: 10.1073/pnas.1006683107. Epub 2010 Jun 7. Proc Natl Acad Sci U S A. 2010. PMID: 20534525 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

