The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome
- PMID: 17640906
- PMCID: PMC1924794
- DOI: 10.1073/pnas.0705408104
The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome
Abstract
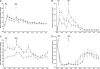
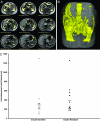
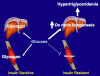
We examined the hypothesis that insulin resistance in skeletal muscle promotes the development of atherogenic dyslipidemia, associated with the metabolic syndrome, by altering the distribution pattern of postprandial energy storage. Following ingestion of two high carbohydrate mixed meals, net muscle glycogen synthesis was reduced by approximately 60% in young, lean, insulin-resistant subjects compared with a similar cohort of age-weight-body mass index-activity-matched, insulin-sensitive, control subjects. In contrast, hepatic de novo lipogenesis and hepatic triglyceride synthesis were both increased by >2-fold in the insulin-resistant subjects. These changes were associated with a 60% increase in plasma triglyceride concentrations and an approximately 20% reduction in plasma high-density lipoprotein concentrations but no differences in plasma concentrations of TNF-alpha, IL-6, adiponectin, resistin, retinol binding protein-4, or intraabdominal fat volume. These data demonstrate that insulin resistance in skeletal muscle, due to decreased muscle glycogen synthesis, can promote atherogenic dyslipidemia by changing the pattern of ingested carbohydrate away from skeletal muscle glycogen synthesis into hepatic de novo lipogenesis, resulting in an increase in plasma triglyceride concentrations and a reduction in plasma high-density lipoprotein concentrations. Furthermore, insulin resistance in these subjects was independent of changes in the plasma concentrations of TNF-alpha, IL-6, high-molecular-weight adiponectin, resistin, retinol binding protein-4, or intraabdominal obesity, suggesting that these factors do not play a primary role in causing insulin resistance in the early stages of the metabolic syndrome.
Conflict of interest statement
Conflict of interest statement: B.B.K. has a research grant from Takeda Pharmaceuticals and is an inventor on a patent for RBP-4.
Figures


References
-
- Reaven GM. Diabetes. 1988;37:1595–1607. - PubMed
-
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Circulation. 2004;109:433–438. - PubMed
-
- Previs SF, Hazey JW, Diraison F, Beylot M, David F, Brunengraber H. J Mass Spectrom. 1996;31:639–642. - PubMed
-
- Diraison F, Pachiaudi C, Beylot M. J Mass Spectrom. 1997;32:81–86. - PubMed
-
- Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, Kissebah AH. Ann Intern Med. 1989;110:867–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR 00125/RR/NCRR NIH HHS/United States
- R01 EB006494/EB/NIBIB NIH HHS/United States
- P01 DK068229/DK/NIDDK NIH HHS/United States
- P01 DK 068229/DK/NIDDK NIH HHS/United States
- R01 DK 43051/DK/NIDDK NIH HHS/United States
- R01 AG023686/AG/NIA NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R01 DK043051/DK/NIDDK NIH HHS/United States
- R01 EB 006494/EB/NIBIB NIH HHS/United States
- R01 AG 23686/AG/NIA NIH HHS/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- P30 DK 45735/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

