Sodium channels amplify spine potentials
- PMID: 17640908
- PMCID: PMC1924793
- DOI: 10.1073/pnas.0705282104
Sodium channels amplify spine potentials
Abstract
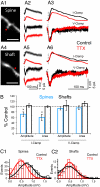
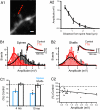
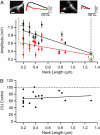
Dendritic spines mediate most excitatory synapses in the brain. Past theoretical work and recent experimental evidence have suggested that spines could contain sodium channels. We tested this by measuring the effect of the sodium channel blocker tetrodotoxin (TTX) on depolarizations generated by two-photon uncaging of glutamate on spines from mouse neocortical pyramidal neurons. In practically all spines examined, uncaging potentials were significantly reduced by TTX. This effect was postsynaptic and spatially localized to the spine and occurred with uncaging potentials of different amplitudes and in spines of different neck lengths. Our data confirm that spines from neocortical pyramidal neurons are electrically isolated from the dendrite and indicate that they have sodium channels and are therefore excitable structures. Spine sodium channels could boost synaptic potentials and facilitate action potential backpropagation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ramón y, Cajal S. La Textura del Sistema Nervioso del Hombre y los Vertebrados. Madrid: Moya; 1899. Primera Edicion.
-
- Chang HT. Cold Spring Harbor Symp Quant Biol. 1952;17:189–202. - PubMed
-
- Llinás R, Hillman DE. In: Neurobiology of Cerebellar Evolution and Development. Llinas R, editor. Chicago: American Medical Association Education and Research Foundation; 1969. pp. 43–73.
-
- Diamond J, Gray EG, Yasargil GM. In: Excitatory Mechanisms, Proc 5th Internation Meeting of Neurobiologists. Andersen P, Jansen J, editors. Oslo: Universitets Forlaget; 1970. pp. 213–222.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

