Epigenetic characterization of hematopoietic stem cell differentiation using miniChIP and bisulfite sequencing analysis
- PMID: 17640913
- PMCID: PMC1924790
- DOI: 10.1073/pnas.0704468104
Epigenetic characterization of hematopoietic stem cell differentiation using miniChIP and bisulfite sequencing analysis
Abstract
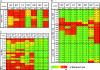
Hematopoietic stem cells (HSC) produce all blood cell lineages by virtue of their capacity to self-renew and differentiate into progenitors with decreasing cellular potential. Recent studies suggest that epigenetic mechanisms play an important role in controlling stem cell potency and cell fate decisions. To investigate this hypothesis in HSC, we have modified the conventional chromatin immunoprecipitation assay allowing for the analysis of 50,000 prospectively purified stem and progenitor cells. Together with bisulfite sequencing analysis, we found that methylated H3K4 and AcH3 and unmethylated CpG dinucleotides colocalize across defined regulatory regions of lineage-affiliated genes in HSC. These active epigenetic histone modifications either accumulated or were replaced by increased DNA methylation and H3K27 trimethylation in committed progenitors consistent with gene expression. We also observed bivalent histone modifications at a lymphoid-affiliated gene in HSC and downstream transit-amplifying progenitors. Together, these data support a model in which epigenetic modifications serve as an important mechanism to control HSC multipotency.
Conflict of interest statement
Conflict of interest statement: I.L.W. has stock in Amgen, Inc., was a member of the Amgen scientific advisory board, and is a cofounder and member of the Board of Directors of Cellerant, Inc., and Stem Cells, Inc.
Figures

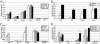

References
-
- Shizuru JA, Negrin RS, Weissman IL. Annu Rev Med. 2005;56:509–538. - PubMed
-
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Annu Rev Immunol. 2003;21:759–806. - PubMed
-
- de Haan G, Gerrits A, Bystrykh L. Curr Opin Hematol. 2006;13:249–253. - PubMed
-
- Loose M, Patient R. Curr Opin Hematol. 2006;13:229–236. - PubMed
-
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. Science. 2002;298:601–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

