Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide
- PMID: 17640917
- PMCID: PMC1940330
- DOI: 10.1073/pnas.0701549104
Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide
Abstract
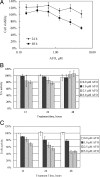
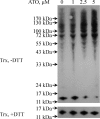
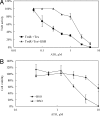
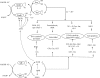
Arsenic trioxide (ATO) is an effective cancer therapeutic drug for acute promyelocytic leukemia and has potential anticancer activity against a wide range of solid tumors. ATO exerts its effect mainly through elevated oxidative stress, but the exact molecular mechanism remains elusive. The thioredoxin (Trx) system comprising NADPH, thioredoxin reductase (TrxR), and Trx and the glutathione (GSH) system composed of NADPH, glutathione reductase, and GSH supported by glutaredoxin are the two electron donor systems that control cellular proliferation, viability, and apoptosis. Recently, the selenocysteine-dependent TrxR enzyme has emerged as an important molecular target for anticancer drug development. Here, we have discovered that ATO irreversibly inhibits mammalian TrxR with an IC(50) of 0.25 microM. Both the N-terminal redox-active dithiol and the C-terminal selenothiol-active site of reduced TrxR may participate in the reaction with ATO. The inhibition of MCF-7 cell growth by ATO was correlated with irreversible inactivation of TrxR, which subsequently led to Trx oxidation. Furthermore, the inhibition of TrxR by ATO was attenuated by GSH, and GSH depletion by buthionine sulfoximine enhanced ATO-induced cell death. These results strongly suggest that the ATO anticancer activity is by means of a Trx system-mediated apoptosis. Blocking cancer cell DNA replication and repair and induction of oxidative stress by the inhibition of both Trx and GSH systems are suggested as cancer chemotherapeutic strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The thioredoxin antioxidant system.Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. Free Radic Biol Med. 2014. PMID: 23899494 Review.
-
Inhibition of Mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity.Cancer Res. 2006 Apr 15;66(8):4410-8. doi: 10.1158/0008-5472.CAN-05-3310. Cancer Res. 2006. PMID: 16618767
-
Inhibition of both thioredoxin reductase and glutathione reductase may contribute to the anticancer mechanism of TH-302.Biol Trace Elem Res. 2010 Sep;136(3):294-301. doi: 10.1007/s12011-009-8544-1. Epub 2009 Oct 17. Biol Trace Elem Res. 2010. PMID: 19838642
-
Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (-)-epigallocatechin-3-gallate.Free Radic Biol Med. 2010 Dec 15;49(12):2010-8. doi: 10.1016/j.freeradbiomed.2010.09.031. Epub 2010 Oct 14. Free Radic Biol Med. 2010. PMID: 20951799
-
Targeting the Thioredoxin System for Cancer Therapy.Trends Pharmacol Sci. 2017 Sep;38(9):794-808. doi: 10.1016/j.tips.2017.06.001. Epub 2017 Jun 22. Trends Pharmacol Sci. 2017. PMID: 28648527 Review.
Cited by
-
Role of glutathione in cancer progression and chemoresistance.Oxid Med Cell Longev. 2013;2013:972913. doi: 10.1155/2013/972913. Epub 2013 May 20. Oxid Med Cell Longev. 2013. PMID: 23766865 Free PMC article. Review.
-
Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems.Mol Cell Proteomics. 2013 Nov;12(11):3285-96. doi: 10.1074/mcp.M113.030437. Epub 2013 Aug 14. Mol Cell Proteomics. 2013. PMID: 23946468 Free PMC article.
-
Cyclophosphamide-evoked heart failure involves pronounced co-suppression of cytoplasmic thioredoxin reductase activity and non-protein free thiol level.Eur J Heart Fail. 2009 Feb;11(2):154-62. doi: 10.1093/eurjhf/hfn012. Eur J Heart Fail. 2009. PMID: 19168513 Free PMC article.
-
Heavy metals and metalloids as a cause for protein misfolding and aggregation.Biomolecules. 2014 Feb 25;4(1):252-67. doi: 10.3390/biom4010252. Biomolecules. 2014. PMID: 24970215 Free PMC article. Review.
-
EM23, a natural sesquiterpene lactone, targets thioredoxin reductase to activate JNK and cell death pathways in human cervical cancer cells.Oncotarget. 2016 Feb 9;7(6):6790-808. doi: 10.18632/oncotarget.6828. Oncotarget. 2016. PMID: 26758418 Free PMC article.
References
-
- Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, et al. Blood. 1996;88:1052–1061. - PubMed
-
- Hu J, Fang J, Dong Y, Chen SJ, Chen Z. Anticancer Drugs. 2005;16:119–127. - PubMed
-
- Gazitt Y, Akay C. Hematology. 2005;10:205–213. - PubMed
-
- Douer D, Tallman MS. J Clin Oncol. 2005;23:2396–2410. - PubMed
-
- Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Cancer Res. 2002;62:3893–3903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

