The continuing case for the Renshaw cell
- PMID: 17640932
- PMCID: PMC2277064
- DOI: 10.1113/jphysiol.2007.136200
The continuing case for the Renshaw cell
Abstract
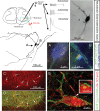
Renshaw cell properties have been studied extensively for over 50 years, making them a uniquely well-defined class of spinal interneuron. Recent work has revealed novel ways to identify Renshaw cells in situ and this in turn has promoted a range of studies that have determined their ontogeny and organization of synaptic inputs in unprecedented detail. In this review we illustrate how mature Renshaw cell properties and connectivity arise through a combination of activity-dependent and genetically specified mechanisms. These new insights should aid the development of experimental strategies to manipulate Renshaw cells in spinal circuits and clarify their role in modulating motor output.
Figures




References
-
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:150–170. - PubMed
-
- Alvarez FJ, Gonzalez-Forero D. Maturation of GABAA synapses on Renshaw cells. Abstr Soc Neurosci. 2006:716–717.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

