Low-affinity spermine block mediating outward currents through Kir2.1 and Kir2.2 inward rectifier potassium channels
- PMID: 17640933
- PMCID: PMC2277198
- DOI: 10.1113/jphysiol.2007.136028
Low-affinity spermine block mediating outward currents through Kir2.1 and Kir2.2 inward rectifier potassium channels
Abstract
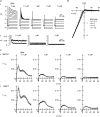
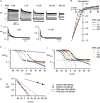
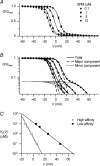
The outward component of the strong inward rectifier K(+) current (I(Kir)) plays a pivotal role in polarizing the membranes of excitable and non-excitable cells and is regulated by voltage-dependent channel block by internal cations. Using the Kir2.1 channel, we previously showed that a small fraction of the conductance susceptible only to a low-affinity mode of block likely carries a large portion of the outward current. To further examine the relevance of the low-affinity block to outward I(Kir) and to explore its molecular mechanism, we studied the block of the Kir2.1 and Kir2.2 channels by spermine, which is the principal Kir2 channel blocker. Current-voltage relations of outward Kir2.2 currents showed a peak, a plateau and two peaks in the presence of 10, 1 and 0.1 microm spermine, respectively, which was explained by the presence of two conductances that differ in their susceptibility to spermine block. When the current-voltage relations showed one peak, like those of native I(Kir), outward Kir2.2 currents were mediated mostly by the conductance susceptible to the low-affinity block. They also flowed in a narrower range than the corresponding Kir2.1 currents, because of 3- to 4-fold greater susceptibility to the low-affinity block than in Kir2.1. Reducing external [K(+)] shifted the voltage dependences of both the high- and low-affinity block of Kir2.1 in parallel with the shift in the reversal potential, confirming the importance of the low-affinity block in mediating outward I(Kir). When Kir2.1 mutants known to have reduced sensitivity to internal blockers were examined, the D172N mutation in the transmembrane pore region made almost all of the conductance susceptible only to low-affinity block, while the E224G mutation in the cytoplasmic pore region reduced the sensitivity to low-affinity block without markedly altering that to the high-affinity block or the high/low conductance ratio. The effects of these mutations support the hypothesis that Kir2 channels exist in two states having different susceptibilities to internal cationic blockers.
Figures




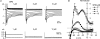

References
-
- Akiyama T, Fozzard HA. Influence of potassium ions and osmolality on the resting membrane potential of rabbit ventricular papillary muscle with estimation of the activity and the activity coefficient of internal potassium. Circ Res. 1975;37:621–629. - PubMed
-
- Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JM. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–1339. - PubMed
-
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. - PubMed
-
- Fakler B, Brandle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

