Oncostatin M gene therapy attenuates liver damage induced by dimethylnitrosamine in rats
- PMID: 17640959
- PMCID: PMC1959500
- DOI: 10.2353/ajpath.2007.060972
Oncostatin M gene therapy attenuates liver damage induced by dimethylnitrosamine in rats
Abstract
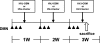
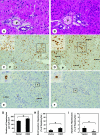
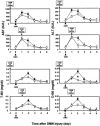
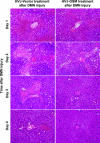
To assess the usefulness of oncostatin M (osm) gene therapy in liver regeneration, we examined whether the introduction of OSM cDNA enhances the regeneration of livers damaged by dimethylnitrosamine (DMN) in rats. Repeated injection of OSM cDNA enclosed in hemagglutinating virus of Japan envelope into the spleen resulted in the exclusive expression of OSM protein in Kupffer cells of the liver, which was accompanied by increases in body weight, liver weight, and serum albumin levels and the reduction of serum liver injury parameters (bilirubin, aspartate aminotransferase, and alanine aminotransferase) and a serum fibrosis parameter (hyaluronic acid). Histological examination showed that osm gene therapy reduced centrilobular necrosis and inflammatory cell infiltration and augmented hepatocyte proliferation. The apoptosis of hepatocytes and fibrosis were suppressed by osm gene therapy. Time-course studies on osm gene therapy before or after DMN treatment showed that this therapy was effective not only in enhancing regeneration of hepatocytes damaged by DMN but in preventing hepatic cytotoxicity caused by subsequent treatment with DMN. These results indicate that OSM is a key mediator for proliferation and anti-apoptosis of hepatocytes and suggest that osm gene therapy is useful, as preventive and curative means, for the treatment of patients with liver damage.
Figures

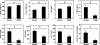


References
-
- Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527–1536. - PubMed
-
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. - PubMed
-
- Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991;13:743–750. - PubMed
-
- Webber EM, FitzGerald MJ, Brown PI, Bartlett MH, Fausto N. Transforming growth factor-alpha expression during liver regeneration after partial hepatectomy and toxic injury, and potential interactions between transforming growth factor-alpha and hepatocyte growth factor. Hepatology. 1993;18:1422–1431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

