The role of complement in inflammatory diseases from behind the scenes into the spotlight
- PMID: 17640961
- PMCID: PMC1959484
- DOI: 10.2353/ajpath.2007.070166
The role of complement in inflammatory diseases from behind the scenes into the spotlight
Abstract
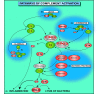
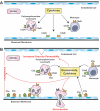
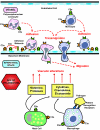
Our understanding of the biology of the complement system has undergone a drastic metamorphosis since its original discovery. This system, which was traditionally primarily described as a "complement" to humoral immunity, is now perceived as a central constituent of innate immunity, defending the host against pathogens, coordinating various events during inflammation, and bridging innate and adaptive immune responses. Complement is an assembly of proteins found in the blood and body fluids and on cell surfaces. Soluble complement components form the proteolytic cascade, whose activation leads to the generation of complement effectors that target various cells involved in the immune response. Membrane-bound receptors and regulators transmit signals from complement effectors to target cells and limit complement activation to the surfaces of pathogens and damaged or activated host cells. The multiple interconnections among complement proteins, immune cells, and mediators provide an excellent mechanism to protect the organism against infections and support the repair of damaged tissues. However, disturbances in this "defense machinery" contribute to the pathogenesis of various diseases. The role of complement in various inflammatory disorders is multifaceted; for example, the activation of complement can significantly contribute to inflammation-mediated tissue damage, whereas inherited or acquired complement deficiencies highly favor the development of autoimmunity.
Figures
References
-
- Bordet J, Gengou O. Sur l’existences de substance sensibilisatrices dans la plupart des serum antimicrobiens. French. Ann Inst Pasteur. 1901;15:289–302.
-
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. - PubMed
-
- Mastellos D, Lambris JD. Cross-disciplinary research stirs new challenges into the study of the structure, function and systems biology of complement. Lambris JD, editor. New York: Springer Science and Business Media, LLC,; Current Topics in Complement. 2006:pp 1–16. - PubMed
-
- Mastellos D, Morikis D, Isaacs SN, Holland MC, Strey CW, Lambris JD. Complement: structure, functions, evolution, and viral molecular mimicry. Immunol Res. 2003;27:367–385. - PubMed
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

