RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth
- PMID: 17640970
- PMCID: PMC1959489
- DOI: 10.2353/ajpath.2007.070049
RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth
Abstract
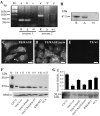
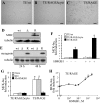
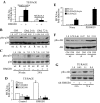
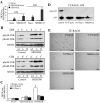
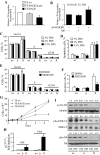
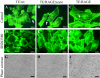
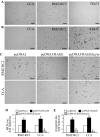
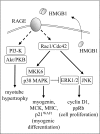
Activation of receptor for advanced glycation end products (RAGE) by its ligand, HMGB1, stimulates myogenesis via a Cdc42-Rac1-MKK6-p38 mitogen-activated protein kinase pathway. In addition, functional inactivation of RAGE in myoblasts results in reduced myogenesis, increased proliferation, and tumor formation in vivo. We show here that TE671 rhabdomyosarcoma cells, which do not express RAGE, can be induced to differentiate on transfection with RAGE (TE671/RAGE cells) but not a signaling-deficient RAGE mutant (RAGEDeltacyto) (TE671/RAGEDeltacyto cells) via activation of a Cdc42-Rac1-MKK6-p38 pathway and that TE671/RAGE cell differentiation depends on RAGE engagement by HMGB1. TE671/RAGE cells also show p38-dependent inactivation of extracellular signal-regulated kinases 1 and 2 and c-Jun NH(2) terminal protein kinase and reduced proliferation, migration, and invasiveness and increased apoptosis, volume, and adhesiveness in vitro; they also grow smaller tumors and show a lower tumor incidence in vivo compared with wild-type cells. Two other rhabdomyosarcoma cell lines that express RAGE, CCA and RMZ-RC2, show an inverse relationship between the level of RAGE expression and invasiveness in vitro and exhibit reduced myogenic potential and enhanced invasive properties in vitro when transfected with RAGEDeltacyto. The rhabdomyosarcoma cell lines used here and C2C12 myoblasts express and release HMGB1, which activates RAGE in an autocrine manner. These data suggest that deregulation of RAGE expression in myoblasts might concur in rhabdomyosarcomagenesis and that increasing RAGE expression in rhabdomyosarcoma cells might reduce their tumor potential.
Figures

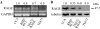

Similar articles
-
The amphoterin (HMGB1)/receptor for advanced glycation end products (RAGE) pair modulates myoblast proliferation, apoptosis, adhesiveness, migration, and invasiveness. Functional inactivation of RAGE in L6 myoblasts results in tumor formation in vivo.J Biol Chem. 2006 Mar 24;281(12):8242-53. doi: 10.1074/jbc.M509436200. Epub 2006 Jan 9. J Biol Chem. 2006. PMID: 16407300
-
Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding.Mol Cell Biol. 2004 Jun;24(11):4880-94. doi: 10.1128/MCB.24.11.4880-4894.2004. Mol Cell Biol. 2004. PMID: 15143181 Free PMC article.
-
Cross-talk between MAP kinase pathways is involved in IGF-independent, IGFBP-6-induced Rh30 rhabdomyosarcoma cell migration.J Cell Physiol. 2010 Sep;224(3):636-43. doi: 10.1002/jcp.22156. J Cell Physiol. 2010. PMID: 20432455
-
Differential effects between amphoterin and advanced glycation end products on colon cancer cells.Int J Cancer. 2003 May 10;104(6):722-7. doi: 10.1002/ijc.11016. Int J Cancer. 2003. PMID: 12640679
-
Rb-mediated apoptosis or proliferation: It's up to JNK.Cell Cycle. 2016;15(1):11-2. doi: 10.1080/15384101.2015.1119492. Cell Cycle. 2016. PMID: 26588003 Free PMC article. Review. No abstract available.
Cited by
-
Spatial and temporal changes in myogenic protein expression by the microenvironment after freeze injury.J Anat. 2019 Mar;234(3):359-367. doi: 10.1111/joa.12925. Epub 2019 Jan 18. J Anat. 2019. PMID: 30657171 Free PMC article.
-
Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression.Int J Mol Sci. 2020 May 20;21(10):3613. doi: 10.3390/ijms21103613. Int J Mol Sci. 2020. PMID: 32443845 Free PMC article. Review.
-
S100B engages RAGE or bFGF/FGFR1 in myoblasts depending on its own concentration and myoblast density. Implications for muscle regeneration.PLoS One. 2012;7(1):e28700. doi: 10.1371/journal.pone.0028700. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276098 Free PMC article.
-
The receptor RAGE: Bridging inflammation and cancer.Cell Commun Signal. 2009 May 8;7:12. doi: 10.1186/1478-811X-7-12. Cell Commun Signal. 2009. PMID: 19426472 Free PMC article.
-
Knockdown of HMGB1 improves apoptosis and suppresses proliferation and invasion of glioma cells.Chin J Cancer Res. 2014 Dec;26(6):658-68. doi: 10.3978/j.issn.1000-9604.2014.12.05. Chin J Cancer Res. 2014. PMID: 25561763 Free PMC article.
References
-
- Arnold HH, Winter B. Muscle differentiation: more complexity to the network of myogenic regulators. Curr Opin Genet Dev. 1998;8:539–544. - PubMed
-
- Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. - PubMed
-
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16:525–532. - PubMed
-
- Lassar AB, Skapek SX, Novitch B. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr Opin Cell Biol. 1994;6:788–794. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

