Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function
- PMID: 17641100
- PMCID: PMC2740819
- DOI: 10.1530/REP-07-0065
Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function
Abstract
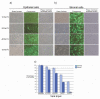
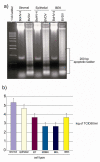
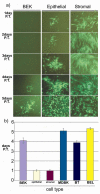
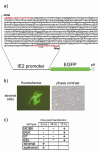
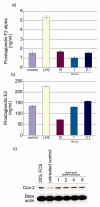
Bovine postpartum uterine disease, metritis, affects about 40% of animals and is widely considered to have a bacterial aetiology. Although the gamma-herpesvirus bovine herpesvirus 4 (BoHV-4) has been isolated from several outbreaks of metritis or abortion, the role of viruses in endometrial pathology and the mechanisms of viral infection of uterine cells are often ignored. The objectives of the present study were to explore the interaction, tropism and outcomes of BoHV-4 challenge of endometrial stromal and epithelial cells. Endometrial stromal and epithelial cells were purified and infected with a recombinant BoHV-4 carrying an enhanced green fluorescent protein (EGFP) expression cassette to monitor the establishment of infection. BoHV-4 efficiently infected both stromal and epithelial cells, causing a strong non-apoptotic cytopathic effect, associated with robust viral replication. The crucial step for the BoHV-4 endometriotropism appeared to be after viral entry as there was enhanced transactivation of the BoHV-4 immediate early 2 gene promoter following transient transfection into the endometrial cells. Infection with BoHV-4 increased cyclooxygenase 2 protein expression and prostaglandin estradiol secretion in endometrial stromal cells, but not epithelial cells. Bovine macrophages are persistently infected with BoHV-4, and co-culture with endometrial stromal cells reactivated BoHV-4 replication in the persistently infected macrophages, suggesting a symbiotic relationship between the cells and virus. In conclusion, the present study provides evidence of cellular and molecular mechanisms, supporting the concept that BoHV-4 is a pathogen associated with uterine disease.
Figures


Similar articles
-
The chemokine IL8 is up-regulated in bovine endometrial stromal cells by the BoHV-4 IE2 gene product, ORF50/Rta: a step ahead toward a mechanism for BoHV-4 induced endometritis.Biol Reprod. 2010 Dec;83(6):919-28. doi: 10.1095/biolreprod.110.086074. Epub 2010 Aug 18. Biol Reprod. 2010. PMID: 20720165
-
Interferon gamma-mediated BoHV-4 replication restriction in bovine endometrial stromal cells is host IDO1 gene expression independent and BoHV-4 IE2 gene expression dependent.Biol Reprod. 2014 Nov;91(5):112. doi: 10.1095/biolreprod.114.123000. Epub 2014 Oct 1. Biol Reprod. 2014. PMID: 25273529
-
Bacterial infection of endometrial stromal cells influences bovine herpesvirus 4 immediate early gene activation: a new insight into bacterial and viral interaction for uterine disease.Reproduction. 2008 Sep;136(3):361-6. doi: 10.1530/REP-08-0171. Epub 2008 Jun 24. Reproduction. 2008. PMID: 18577555 Free PMC article.
-
[Bovine herpesvirus 4 (BoHV-4): general aspects of the biology and status in Argentina].Rev Argent Microbiol. 2015 Apr-Jun;47(2):155-66. doi: 10.1016/j.ram.2015.02.007. Epub 2015 May 9. Rev Argent Microbiol. 2015. PMID: 25962539 Review. Spanish.
-
Biology of bovine herpesvirus 5.Vet J. 2010 May;184(2):138-45. doi: 10.1016/j.tvjl.2009.03.035. Epub 2009 May 5. Vet J. 2010. PMID: 19409823 Review.
Cited by
-
Isolation and characterization of bovine herpesvirus 4 (BoHV-4) from a cow affected by post partum metritis and cloning of the genome as a bacterial artificial chromosome.Reprod Biol Endocrinol. 2009 Aug 19;7:83. doi: 10.1186/1477-7827-7-83. Reprod Biol Endocrinol. 2009. PMID: 19691825 Free PMC article.
-
Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model.Cells. 2022 Jun 7;11(12):1858. doi: 10.3390/cells11121858. Cells. 2022. PMID: 35740987 Free PMC article. Review.
-
Role of Genital Tract Bacteria in Promoting Endometrial Health in Cattle.Microorganisms. 2022 Nov 12;10(11):2238. doi: 10.3390/microorganisms10112238. Microorganisms. 2022. PMID: 36422307 Free PMC article.
-
Improved filtration method to isolate pure populations of primary bovine endometrial epithelial and stromal cells for immunological studies.Vet Res Commun. 2020 Feb;44(1):29-39. doi: 10.1007/s11259-020-09770-3. Epub 2020 Feb 21. Vet Res Commun. 2020. PMID: 32086740 Free PMC article.
-
First detection and impact of bovine herpesvirus type 4 on dairy cattle reproduction in Thailand.Vet World. 2024 Oct;17(10):2259-2266. doi: 10.14202/vetworld.2024.2259-2266. Epub 2024 Oct 7. Vet World. 2024. PMID: 39619933 Free PMC article.
References
-
- Allsopp TE, Fazakerley JK. Altruistic cell suicide and the specialized case of the virus-infected nervous system. Trends in Neuroscience. 2000;23:284–90. - PubMed
-
- Appleton I, Tomlinson A, Willoughby DA. Induction of cyclo-oxygenase and nitric oxide synthase in inflammation. Advances in Pharmacology. 1996;35:27–78. - PubMed
-
- Arosh JA, Parent J, Chapdelaine P, Sirois J, Fortier MA. Expression of cyclooxygenases 1 and 2 and prostaglandin E synthase in bovine endometrial tissue during the estrous cycle. Biology of Reproduction. 2002;67:161–169. - PubMed
-
- Asselin E, Goff AK, Bergeron H, Fortier MA. Influence of sex steroids on the production of prostaglandins F2 alpha and E2 and response to oxytocin in cultured epithelial and stromal cells of the bovine endometrium. Biology of Reproduction. 1996;54:371–379. - PubMed
-
- Bartha A, Juhasz M, Liebermann H. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjuntivitis. Acta Veterinaria Academiae Scientiarum Hungaricae. 1966;16:357–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

