Early interstitial lung disease in familial pulmonary fibrosis
- PMID: 17641157
- PMCID: PMC1994234
- DOI: 10.1164/rccm.200702-254OC
Early interstitial lung disease in familial pulmonary fibrosis
Abstract
Rationale: Identification of early, asymptomatic interstitial lung disease (ILD) in populations at risk of developing idiopathic pulmonary fibrosis (IPF) may improve the understanding of the natural history of IPF.
Objectives: To determine clinical, radiographic, physiologic, and pathologic features of asymptomatic ILD in family members of patients with familial IPF.
Methods: One hundred sixty-four subjects from 18 kindreds affected with familial IPF were evaluated for ILD. Bronchoalveolar lavage fluid cells were analyzed using flow cytometry. Lung biopsies were performed in six subjects with asymptomatic ILD.
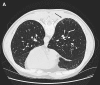
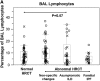
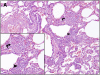
Measurements and main results: High-resolution computed tomography abnormalities suggesting ILD were identified in 31 (22%) of 143 asymptomatic subjects. Subjects with asymptomatic ILD were significantly younger than subjects with known familial IPF (P < 0.001) and significantly older than related subjects without lung disease (P < 0.001). A history of smoking was identified in 45% of subjects with asymptomatic ILD and in 67% of subjects with familial IPF; these percentages were significantly higher than that of related subjects without lung disease (23%) (P = 0.02 and P < 0.001, respectively). Percentages of activated CD4(+) lymphocytes were significantly higher in bronchoalveolar lavage fluid cells from subjects with asymptomatic ILD compared with related subjects without lung disease (P < 0.001). Lung biopsies performed in subjects with asymptomatic ILD revealed diverse histologic subtypes.
Conclusions: Asymptomatic ILD in individuals at risk of developing familial IPF can be identified using high-resolution computed tomography scan of the chest, especially in those with a history of smoking. Lung biopsies from individuals in this cohort with early asymptomatic lung disease demonstrate various histologic subtypes of ILD.
Figures













References
-
- Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med 2000;161:1172–1178. - PubMed
-
- Collard HR, Ryu JH, Douglas WW, Schwarz MI, Curran-Everett D, King TE Jr, Brown KK. Combined corticosteroid and cyclophosphamide therapy does not alter survival in idiopathic pulmonary fibrosis. Chest 2004;125:2169–2174. - PubMed
-
- Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE Jr. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. - PubMed
-
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001;345:517–525. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

