Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke
- PMID: 17641243
- PMCID: PMC2792206
- DOI: 10.1634/stemcells.2007-0169
Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke
Abstract
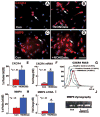
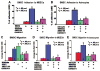
Stromal cell-derived factor-1 (SDF1) and its chemokine (CXC motif) receptor 4 (CXCR4), along with matrix metalloproteinases (MMPs), regulate bone marrow stromal cell (BMSC) migration. We tested the hypothesis that a nitric oxide donor, DETA-NONOate, increases endogenous ischemic brain SDF1 and BMSC CXCR4 and MMP9 expression, which promotes BMSC migration into ischemic brain and thereby enhances functional outcome after stroke. C57BL/6J mice were subjected to middle cerebral artery occlusion (MCAo), and 24 hours later, the following were intravenously administered (n = 9 mice per group): (a) phosphate-buffered saline; (b) BMSCs (5 x 10(5)); (c) 0.4 mg/kg DETA-NONOate; (d) combination of CXCR4-inhibition BMSCs with DETA-NONOate; and (e) combination of BMSCs with DETA-NONOate. To elucidate the mechanisms underlying combination-enhanced BMSC migration, transwell cocultures of BMSC with mouse brain endothelial cells (MBECs) or astrocytes were performed. Combination treatment significantly improved functional outcome after stroke compared with BMSC monotherapy and MCAo control, and it increased SDF1 expression in the ischemic brain compared with DETA-NONOate monotherapy and MCAo control. The number of BMSCs in the ischemic brain was significantly increased after combination BMSC with DETA-NONOate treatment compared with monotherapy with BMSCs. The number of engrafted BMSCs was significantly correlated with functional outcome after stroke. DETA-NONOate significantly increased BMSC CXCR4 and MMP9 expression and promoted BMSC adhesion and migration to MBECs and astrocytes compared with nontreatment BMSCs. Inhibition of CXCR4 or MMPs in BMSCs significantly decreased DETA-NONOate-induced BMSC adhesion and migration. Our data demonstrate that DETA-NONOate enhanced the therapeutic potency of BMSCs, possibly via upregulation of SDF1/CXCR4 and MMP pathways, and increased BMSC engraftment into the ischemic brain.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures


References
-
- Dezawa M, Hoshino M, Nabeshima Y, et al. Marrow stromal cells: Implications in health and disease in the nervous system. Curr Mol Med. 2005;5:723–732. - PubMed
-
- Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. - PubMed
-
- Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. - PubMed
-
- Muller-Ehmsen J, Krausgrill B, Burst V, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–884. - PubMed
-
- Bagri A, Gurney T, He X, et al. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

