Integrin beta6 mediates phospholipid and collectin homeostasis by activation of latent TGF-beta1
- PMID: 17641300
- PMCID: PMC2219547
- DOI: 10.1165/rcmb.2006-0428OC
Integrin beta6 mediates phospholipid and collectin homeostasis by activation of latent TGF-beta1
Abstract
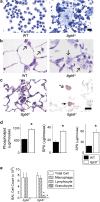
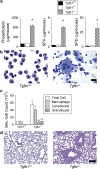
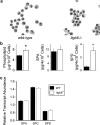
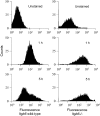
Surfactant lines the alveolar surface and prevents alveolar collapse. Derangements of surfactant cause respiratory failure and interstitial lung diseases. The collectins, surfactant proteins A and D, are also important in innate host defense. However, surfactant regulation in the postnatal lung is poorly understood. We found that the epithelial integrin, alphavbeta6, regulates surfactant homeostasis in vivo by activating latent transforming growth factor (TGF)-beta. Adult mice lacking the beta-subunit of alphavbeta6 (Itgb6-/-) developed increased bronchoalveolar lavage phospholipids and surfactant proteins A and D, and demonstrated abnormal-appearing alveolar macrophages, reminiscent of the human disease pulmonary alveolar proteinosis. Using lung-specific expression of constitutively active TGF-beta1 in Itgb6-/- mice, we found that TGF-beta1 was sufficient to normalize these abnormalities. Tgfbeta1-deficient mice also demonstrated increased phospholipids and surfactant proteins A and D, but mice lacking the key TGF-beta signaling molecule, SMAD3, did not. Therefore, integrin-mediated activation of latent TGF-beta1 regulates surfactant constituents independent of intracellular SMAD3. In vivo increases in surfactant protein A and D were not associated with increases in mRNA for these proteins in alveolar tissue from Itgb6-/- mice. On the other hand, isolated alveolar macrophages from Itgb6-/- mice were defective in processing phospholipids in vitro, suggesting that reduced surfactant clearance contributes to altered surfactant homeostasis in these mice in vivo. These findings show that alphavbeta6 and TGF-beta1 regulate homeostasis of phospholipids and collectins in adult mouse lungs and may have implications for anti-fibrotic therapeutics that inhibit active TGF-beta in the lung.
Figures


Comment in
-
What the lung has taught us about latent TGF-beta activation.Am J Respir Cell Mol Biol. 2008 Nov;39(5):499-502. doi: 10.1165/rcmb.2008-0003ED. Am J Respir Cell Mol Biol. 2008. PMID: 18927350 Free PMC article. Review. No abstract available.
References
-
- Rooney SA, Young SL, Mendelson CR. Molecular and cellular processing of lung surfactant. FASEB J 1994;8:957–967. - PubMed
-
- Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol 2002;64:775–802. - PubMed
-
- Rider ED, Ikegami M, Jobe AH. Localization of alveolar surfactant clearance in rabbit lung cells. Am J Physiol 1992;263:L201–L209. - PubMed
-
- Wright JR. Clearance and recycling of pulmonary surfactant. Am J Physiol 1990;259:L1–L12. - PubMed
-
- Nogee LM. Genetics of pediatric interstitial lung disease. Curr Opin Pediatr 2006;18:287–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

