1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway
- PMID: 17641676
- PMCID: PMC1978255
- DOI: 10.1038/sj.bjp.0707383
1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway
Abstract
Background and purpose: 1-methylnicotinamide (MNA) has been considered to be an inactive metabolite of nicotinamide. Here we assessed the anti-thrombotic activity of MNA in vivo.
Experimental approach: Antithrombotic action of MNA was studied in normotensive rats with extracorporeal thrombus formation (thrombolysis), in renovascular hypertensive rats with intraarterial thrombus formation (arterial thrombosis) and in a venous thrombosis model in rats (venous thrombosis).
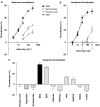
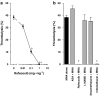
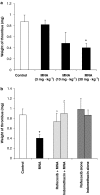
Key results: MNA (3-100 mg kg(-1)) induced a dose-dependent and sustained thrombolytic response, associated with a rise in 6-keto-PGF(1alpha) in blood. Various compounds structurally related to MNA were either inactive or weaker thrombolytics. Rofecoxib (0.01-1 mg kg(-1)), dose-dependently inhibited the thrombolytic response of MNA, indomethacin (5 mg kg(-1)) abolished it, while L-NAME (5 mg kg(-1)) were without effect. MNA (3-30 mg kg(-1)) also reduced arterial thrombosis and this effect was abrogated by indomethacin (2.5 mg kg(-1)) as well as by rofecoxib (1 mg kg(-1)). MNA, however, did not affect venous thrombosis. In vitro MNA did not modify platelet aggregation nor induce vasodilation.
Conclusions and implications: MNA displayed a profile of anti-thrombotic activity in vivo that surpasses that of closely related compounds. MNA inhibited platelet-dependent thrombosis by a mechanism involving cyclooxygenase-2 and prostacyclin. Our findings suggest that endogenous MNA, produced in the liver by nicotinamide N-methyltransferase, could be an endogenous activator of prostacyclin production and thus may regulate thrombotic as well as inflammatory processes in the cardiovascular system.
Figures

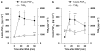
References
-
- Aoyama K, Matsubara K, Okada K, Fukushima S, Shimizu K, Yamaguchi S, et al. N-methylation ability for azaheterocyclic amines is higher in Parkinson's disease: nicotinamide loading test. J Neural Transm. 2000;107:985–995. - PubMed
-
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. - PubMed
-
- Bieron K, Swies J, Kostka-Trabka E, Gryglewski RJ. Thrombolytic and antiplatelet action of xanthinol nicotinate (Sadamin): possible mechanisms. J Physiol Pharmacol. 1998;49:241–249. - PubMed
-
- Chong ZZ, Lin SH, Maiese K. Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J Vasc Res. 2002;39:131–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

