Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease
- PMID: 17641782
- PMCID: PMC1913490
- DOI: 10.1172/JCI31602
Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease
Abstract
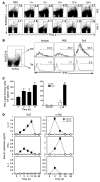
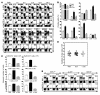
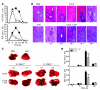
Because of the paucity of known self lipid-reactive ligands for NKT cells, interactions among distinct NKT cell subsets as well as immune consequences following recognition of self glycolipids have not previously been investigated. Here we examined cellular interactions and subsequent immune regulatory mechanism following recognition of sulfatide, a self-glycolipid ligand for a subset of CD1d-restricted type II NKT cells. Using glycolipid/CD1d tetramers and cytokine responses, we showed that activation of sulfatide-reactive type II NKT cells and plasmacytoid DCs caused IL-12- and MIP-2-dependent recruitment of type I, or invariant, NKT (iNKT) cells into mouse livers. These recruited iNKT cells were anergic and prevented concanavalin A-induced (ConA-induced) hepatitis by specifically blocking effector pathways, including the cytokine burst and neutrophil recruitment that follow ConA injection. Hepatic DCs from IL-12(+/+) mice, but not IL-12(-/-) mice, adoptively transferred anergy in recipients; thus, IL-12 secretion by DCs enables them to induce anergy in iNKT cells. Our data reveal what we believe to be a novel mechanism in which interactions among type II NKT cells and hepatic DCs result in regulation of iNKT cell activity that can be exploited for intervention in inflammatory diseases, including autoimmunity and asthma.
Figures





References
-
- Crispe I.N. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 2003;3:51–62. - PubMed
-
- Calne R.Y., et al. Toleragenic effects of porcine liver allografts. Br. J. Surg. 1969;56:692–693. - PubMed
-
- Herkel J., Schuchmann M., Tiegs G., Lohse A.W. Immune-mediated liver injury. J. Hepatol. 2005;42:920–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

