The peptidase inhibitor CGS-26303 increases endothelin converting enzyme-1 expression in endothelial cells through accumulation of big endothelin-1
- PMID: 17643133
- PMCID: PMC2042959
- DOI: 10.1038/sj.bjp.0707398
The peptidase inhibitor CGS-26303 increases endothelin converting enzyme-1 expression in endothelial cells through accumulation of big endothelin-1
Abstract
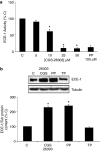
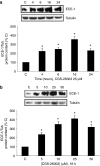
Background and purpose: CGS-26303 inhibits endothelin converting enzyme (ECE)-1 more specifically than phosphoramidon. We have studied the effect of CGS-26303 on ECE-1 expression in bovine aortic endothelial cells.
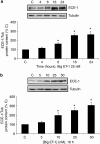
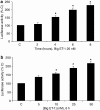
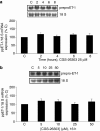
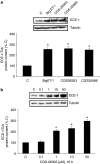
Methods: ECE-1 activity and big endothelin (ET)-1 levels were measured by ELISA, ECE-1 expression using western and northern blot and promoter activity using transfection assays.
Key results: ECE-1 activity was completely inhibited by CGS-26303 25 microM and phosphoramidon 100 microM. CGS-26303 and phosphoramidon, though not thiorphan, a neutral endopeptidase (NEP) inhibitor, stimulated ECE-1 expression in cells (maximal effect at 16 h, 25 microM). Cycloheximide abolished that effect. CGS-26303 induced ECE-1 mRNA expression and ECE-1 promoter activity. CGS-35066, a selective ECE-1 inhibitor, mimicked the effects of CGS-26303, suggesting that the effect was specific to ECE-1 inhibition. Big ET-1 accumulated in the cells and in the supernatants after CGS-26303 treatment. Neither exogenously added ET-1 nor the blockade of their receptors with bosentan modified ECE-1 protein. When big ET-1 was added to cells, significant increases in ECE-1 protein content and ECE-1 promoter activity were found. Bosentan did not block those effects. CGS-26303 did not modify prepro-ET-1 expression. CGS-26303 and big ET-1 induced the same effects in human endothelial cells, at lower doses.
Conclusions: These results suggest that the accumulation of big ET-1 is responsible for the effects of CGS-26303 on ECE-1 and they did not depend on NEP blockade. Changes in ECE-1 protein after the administration of CGS-26303 could lead to a decreased response in long-term treatments.
Figures





References
-
- Aharinejad S, Krenn K, Paulus P, Schafer R, Zuckermann A, Grimm M, et al. Differential role of TGF-beta1/bFGF and ET-1 in graft fibrosis in heart failure patients. Am J Transplant. 2005;5:2185–2192. - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
-
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270:15262–15268. - PubMed
-
- Eto M, Barandier C, Rathgeb L, Kozai T, Joch H, Yang Z, et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res. 2001;89:583–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

