Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme
- PMID: 17643375
- PMCID: PMC4498903
- DOI: 10.1016/j.molcel.2007.06.027
Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme
Abstract
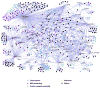
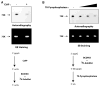
We have performed a survey of soluble human protein complexes containing components of the transcription and RNA processing machineries using protein affinity purification coupled to mass spectrometry. Thirty-two tagged polypeptides yielded a network of 805 high-confidence interactions. Remarkably, the network is significantly enriched in proteins that regulate the formation of protein complexes, including a number of previously uncharacterized proteins for which we have inferred functions. The RNA polymerase II (RNAP II)-associated proteins (RPAPs) are physically and functionally associated with RNAP II, forming an interface between the enzyme and chaperone/scaffolding proteins. BCDIN3 is the 7SK snRNA methylphosphate capping enzyme (MePCE) present in an snRNP complex containing both RNA processing and transcription factors, including the elongation factor P-TEFb. Our results define a high-density protein interaction network for the mammalian transcription machinery and uncover multiple regulatory factors that target the transcription machinery.
Figures




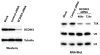
Comment in
-
Transcriptional networking cap-tures the 7SK RNA 5'-gamma-methyltransferase.Mol Cell. 2007 Aug 17;27(4):517-9. doi: 10.1016/j.molcel.2007.08.001. Mol Cell. 2007. PMID: 17707222
References
-
- Aguilera A. Cotranscriptional mRNP assembly: from the DNA to the nuclear pore. Curr Opin Cell Biol. 2005;17:242–250. - PubMed
-
- Baillat D, Hakimi MA, Naar AM, Shilatifard A, Cooch N, Shiekhattar R. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell. 2005;123:265–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

